-
Improving, Retaining, and Separating Polar Compounds Using Chromatographic Techniques
Separating and retaining polar compounds are significant challenges in chromatography. Here, we explore the key technical considerations and solutions for effectively handling these compounds, focusing on advanced chromatographic techniques and column technologies. What Are Polar Compounds? Polar compounds are essential in biological processes, drug design, and industrial applications due to their ability to interact with…

Recent Posts
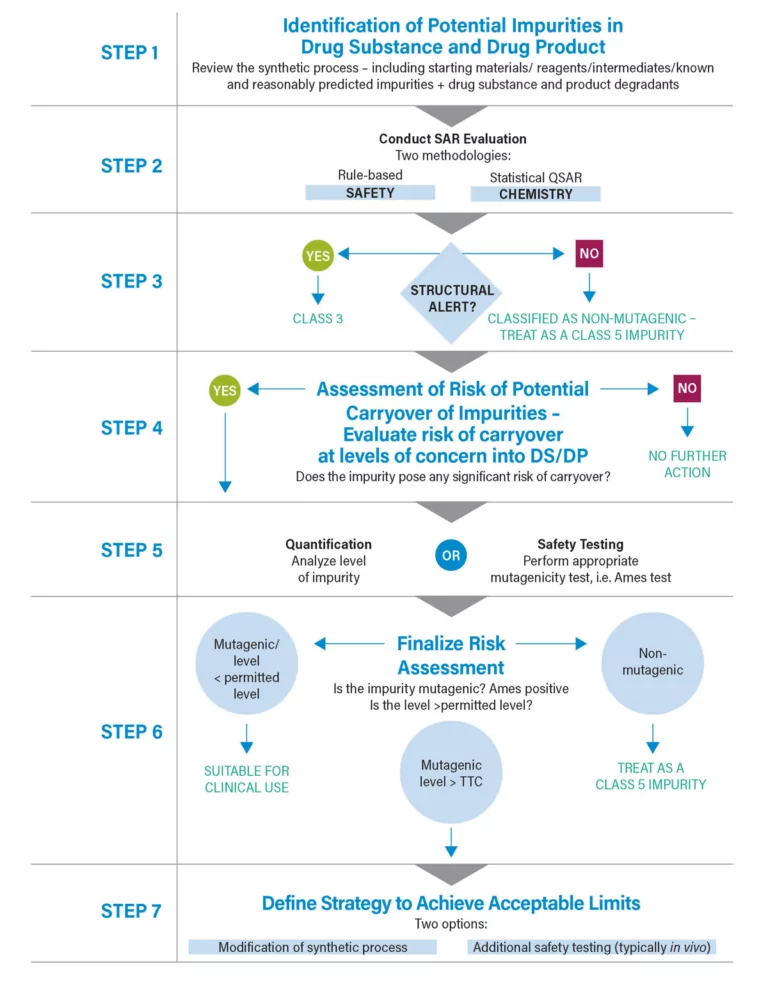
Mutagenic Impurity Risk Assessment Throughout the Development and Manufacturing Process
Since the early 2000s and the advent of the first guidance relating to mutagenic impurities developed by the European Medicines Agency (EMA), it has been necessary to assess the risk posed by mutagenic impurities. Although initially it was thought that avoidance was an option, it quickly became apparent that this was not a viable strategy. ...
Five Questions with Waters’ New President and CEO, Udit Batra
On September 1, 2020, Udit Batra became Waters’ President and CEO. Udit brings a wealth of knowledge and experience to Waters, having previously held executive positions at Novartis and Merck KGaA. Most recently, he served as the CEO of MilliporeSigma, the life science tools business at Merck KGaA. We caught up with Udit during this...
Bringing the Future Into Focus with Waters’ Virtual Events
In the midst of the COVID-19 pandemic the power of technology has emerged center stage, keeping the industry connected at a time when many ordinary avenues of communication have been cut off over safety concerns. In order to drive forward the next generation of technological and research innovations, we understand how vital it is that...
Understanding Sample Complexity: Determining Matrix Effects in Complex Food Samples
While the hyphenation of chromatographic and mass spectrometry technologies has revolutionized food contaminant testing laboratories, one major drawback is the potential for the phenomenon of matrix effects. Due to unwanted interactions between the analytes and sample matrix, the analyte’s response may be reduced or amplified. The influence of matrix on the reliability of your method...
Traceability in OneLab: OneLab configuration by the Lab Administrator
Learn how to bring increased confidence to your sample preparation workflows using automation, smart lab tools and electronic documentation from Waters. This blog post was revised in September 2023 to include the most up-to-date information.
Traceability in OneLab: Protocols Designed by the Expert Analyst
OneLab, cloud-native laboratory software, allows members to securely design and implement sample preparation protocols for lab analysts. Learn more by reading this post, which was revised in September 2023 to include the most up-to-date information.
Popular Topics
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (23) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (21) mass detection (16) mass spectrometry (MS) (54) method development (13) STEM (12) sustainability (12)