USP<787>: Tailored Guidance for Better Biologics

Understanding US<787> and its Impact on Pharmaceutical Quality with Tailored Guidance for Biologics
In the world of pharmaceuticals, quality is paramount. Ensuring the safety and efficacy of medications is not only a regulatory requirement but also a moral obligation to patients. To achieve this, regulatory bodies like the United States Pharmacopeia (USP) lay down guidelines and standards that pharmaceutical companies must adhere to. Two such standards, USP<787> and USP<788>, play a crucial role in maintaining the quality of pharmaceutical products, particularly in relation to particulate matter. Let’s delve into what these standards entail and how they compare.
What is USP<787>?
USP<787>, titled “Subvisible Particulate Matter in Therapeutic Protein Injections,” provides guidelines for assessing the presence of subvisible particulate matter in protein-based pharmaceutical products. These particulates, though not visible to the naked eye, can potentially impact product safety and efficacy. USP<787> outlines specific testing methods and acceptance criteria for detecting and quantifying these particulates, helping manufacturers ensure the quality of their protein injections.
Key Components of USP<787>:
- Testing Methods: USP<787> details various analytical techniques for detecting subvisible particulate matter, including light obscuration, microscopy, and flow imaging analysis. Each method has its advantages and limitations, allowing manufacturers to choose the most suitable approach based on their product characteristics.
- 验收标准:该标准根据颗粒物的尺寸和性质,规定了颗粒物的验收标准。这些标准对于判定产品是否符合质量标准,或需要进一步调查并可能采取纠正措施至关重要。
- Risk Assessment: USP<787> emphasizes the importance of risk assessment in evaluating the impact of particulate matter on product safety and efficacy. Manufacturers are encouraged to conduct thorough risk assessments to identify potential risks associated with particulate contamination and implement appropriate mitigation strategies.
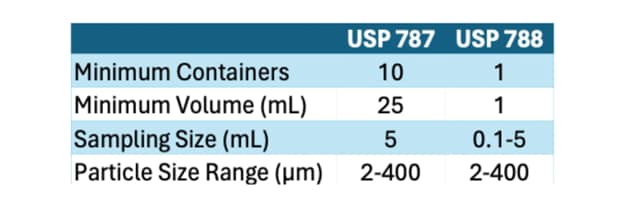
Comparison with USP<788>:
While USP<787> focuses specifically on protein-based pharmaceuticals, USP<788> addresses particulate matter in injections as a whole, encompassing both protein and non-protein formulations. The key differences between the two standards lie in their scope and application:
- Scope: USP<788> is broader in scope, covering all injectable products, including both small-molecule drugs and biologics, whereas USP<787> specifically targets therapeutic protein injections.
- Testing Methods: While both standards prescribe similar testing methods for particulate matter analysis, USP<787> may have additional considerations specific to protein formulations, such as protein aggregation and particle morphology.
- Acceptance Criteria: The acceptance criteria outlined in USP<788> are generally applicable to all injectable products, whereas USP<787> may have more tailored criteria considering the unique characteristics of protein-based formulations.
An important emphasis of USP<787> is that it acknowledges the limitations of light obscuration for protein-based particles and encourages additional methods like microscopy and image analysis for comprehensive characterization.
合规的重要性
Adherence to standards like USP<787> and USP<788> is not just about regulatory compliance; it’s about ensuring patient safety and maintaining the reputation of pharmaceutical products. By following these guidelines, manufacturers can mitigate the risks associated with particulate contamination and uphold the quality of their products throughout the manufacturing process.
USP<787> plays a vital role in ensuring the quality and safety of therapeutic protein injections by providing comprehensive guidelines for detecting and assessing subvisible particulate matter. While similar in essence, USP<788> covers a broader spectrum of injectable products, highlighting the importance of understanding the specific requirements applicable to each product type. Ultimately, compliance with these standards is indispensable for pharmaceutical manufacturers committed to delivering safe and effective medications to patients worldwide.
How to Perform USP<787>
In the realm of pharmaceutical quality assurance, the ability to accurately detect and characterize subvisible particulate matter is paramount. One of the primary methods prescribed by USP<787> for this purpose is light microscopy. Let’s explore how light microscopy serves as a vital tool in the analysis of therapeutic protein injections and why its utilization is crucial for pharmaceutical manufacturers.
光学显微镜的主要优势
- 颗粒表征:光学显微镜能为颗粒物的特性(如尺寸、形状和形态)提供宝贵信息。这些信息对于评估颗粒物对产品安全性和有效性的潜在影响至关重要。
- 高分辨率:现代光学显微镜具备高分辨率成像能力,可对单个颗粒进行精细观察。这种细节水平对于区分不同类型的颗粒物及其来源至关重要。
- 实时分析:与其他分析技术不同,光学显微镜能够实现颗粒的实时观察,使分析过程中可即时作出决策。这种快速反馈机制对于确保高效准确地检测颗粒污染物具有不可估量的价值。
挑战与考量
虽然光学显微镜是分析颗粒物的强大工具,但它确实存在某些必须解决的局限性和挑战:
- 样品制备:正确的样品制备对获得可靠的光学显微镜结果至关重要。必须确保样品得到适当分散和固定,以实现清晰成像。
- 操作员技能:解读显微镜图像需要具备一定专业知识和经验。必须由受过培训的操作员准确识别和表征颗粒物,以最大限度降低误判风险。
- 仪器校准:定期对显微镜设备进行校准和维护,对于确保长期测量准确性和性能可靠性至关重要。
现代自动化光学显微镜技术——Aura系统
与 Aura PTx 系统,所有这些挑战和考量都已解决。 奥拉系统 are based on membrane/filter microscopy and are USP<787> compliant. Utilizing high-contrast imaging techniques, Background Membrane Imaging (BMI) and Fluorescence Membrane Imaging (FMM), drug manufacturers can detect, count, and size particulate matter with high confidence and reproducibility:
- 高通量:通过同时检测多个样本降低样本制备风险。Aura系统每项检测仅需1分钟。
- 全自动化并提供支持 CFR Software: 消除操作员失误与主观偏差。Aura系统实现全自动化运行,无需专业操作员即可采集数据。此外,Particle Vue软件能自动识别样本中的颗粒物,并进行尺寸测量与数量统计。
- 坚固可靠的仪器设备:Aura系统是低维护、高可靠性的仪器。对于需要更周全维护与服务的需求,沃特世提供多种服务/维护方案以及安装确认/运行确认(IQ/OQ)服务。
The Role of Light Microscopy in USP<787> Compliance
In accordance with USP<787> guidelines, pharmaceutical manufacturers rely on light microscopy as a key tool for assessing the presence of subvisible particulate matter in therapeutic protein injections. By leveraging the capabilities of light microscopy, manufacturers can effectively identify and quantify particulate contaminants从而确保符合监管标准,并维护其产品的质量。
In conclusion, light microscopy and Aura PTx Systems play a pivotal role in USP<787> analysis, offering pharmaceutical manufacturers a reliable means of detecting and characterizing subvisible particulate matter in therapeutic protein injections. Despite its challenges, the benefits of using light microscopy for particulate analysis far outweigh the drawbacks, making it an indispensable tool in the quest for pharmaceutical quality assurance.