-
Improving, Retaining, and Separating Polar Compounds Using Chromatographic Techniques
Separating and retaining polar compounds are significant challenges in chromatography. Here, we explore the key technical considerations and solutions for effectively handling these compounds, focusing on advanced chromatographic techniques and column technologies. What Are Polar Compounds? Polar compounds are essential in biological processes, drug design, and industrial applications due to their ability to interact with…

Recent Posts
5 Insights: Protein Quantification Using the Surrogate Peptide Method
What can you get all the top Pharma and CRO's scientists to agree on? That the biopharma industry is growing and quantifying biotherapeutics for bioanalysis is difficult - but you have options.
Developing Separation Methods for Natural Products: Questions and Answers from a Webinar with Dr. Tom Wheat
The development of chromatographic methods for natural products is challenging due to the complexity of the sample matrix and analyte chemistry. However, by understanding the fundamental parameters of your chromatography and strategies for efficient detection, there are many ways to overcome these difficulties. I recently presented a webinar describing some techniques and tools used for...
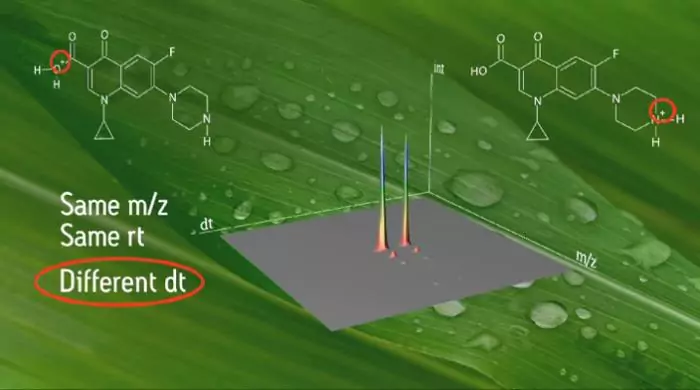
Is Ion Mobility Mass Spectrometry Child’s Play?
On Wednesday evening after dinner and my usual digestif, I was watching the webcast “Making Ion Mobility Mass Spectrometry Routine” when my son walked by. “Wow dad, that’s kind of cool! What the heck is it?” he asked. “It’s ion mobility!! It’s an orthogonal and complementary resource for chromatography and mass spectrometry…” The digestif was...
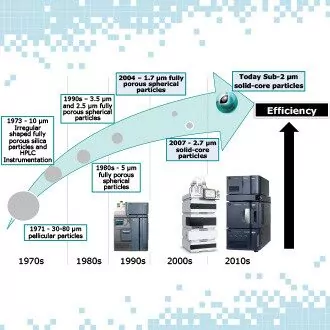
Unleash the maximum separation performance from your solid-core column
Webinar Highlights | Understanding the Impact of System Dispersion on Separation Performance Hi, I am Jonathan E. Turner, Product Marketing Manager for ACQUITY® UPLC® and CORTECS® lines of columns at Waters Corporation. I recently conducted a webinar that talked about the changes we have seen the last 10 years and the substantial improvements in both...
Laboratory Developed Tests: More Oversight, or More Vigilance and Reporting?
U.S. FDA Prepares to Oversee LDTs Recently, the U.S. Food and Drug Administration’s Office of Public Health Strategy and Analysis issued an interesting (and much anticipated) report regarding Laboratory Developed Tests (LDTs). The Agency has consistently cited a need for greater oversight of LDTs out of concern for public safety. Laboratory Developed Tests used to...
Attention Method Development Scientists! Learn about novel tools for replicating your existing methods
Webinar Highlights | Simplifying Methods Transfer: Novel Tools for Replicating Your Established Methods on an ACQUITY Arc System I’m Dr. Paula Hong, Principal Scientist at Waters Corporation. I recently conducted a webinar that talked about the instrument characteristics that can impact transfer of established reversed-phase methods across both HPLC and UHPLC systems. These factors include dwell...
Popular Topics
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (23) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (21) mass detection (16) mass spectrometry (MS) (54) method development (13) STEM (12) sustainability (12)