-
Protecting Your Laboratory Data: Cybersecurity at Waters
With the ever-present threat of cyberattacks, safeguarding laboratory data has become a top priority for scientists across the globe. Many labs are accelerating digitalization efforts, but that could expose their data to greater risks. The Increase in Lab Digitalization: Effects on Cybersecurity As it turns out, the increase in digitalization, particularly the shift to the…

Recent Posts
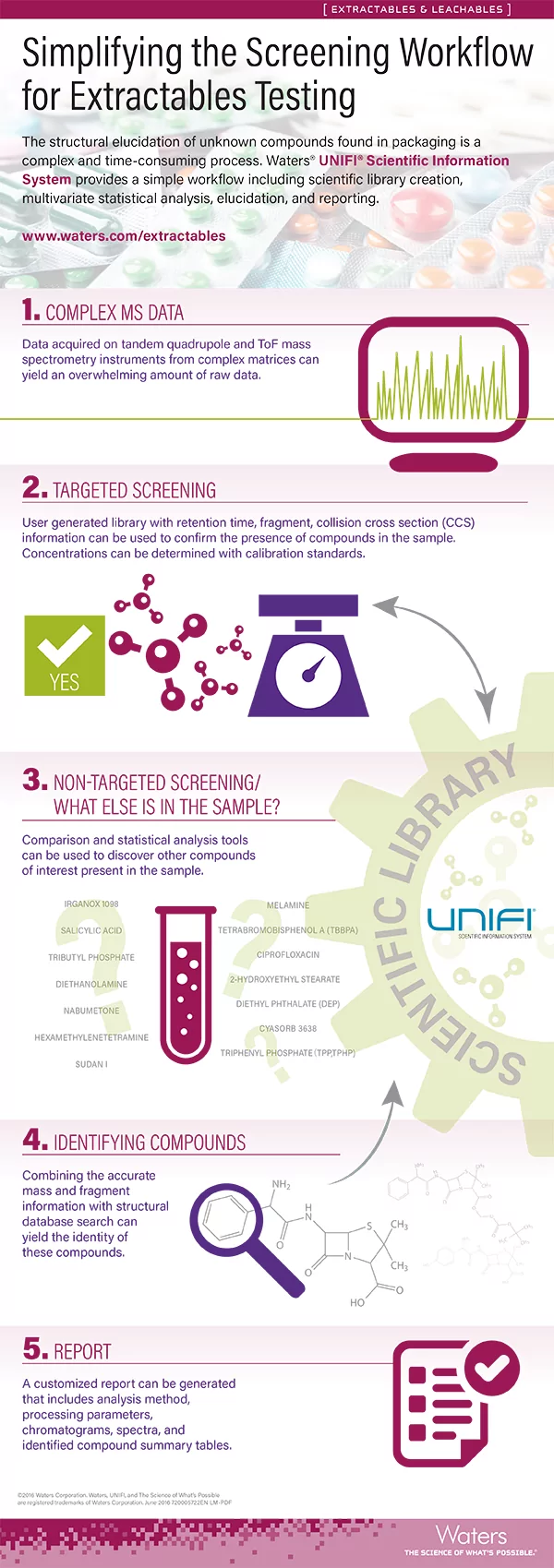
Infographic: Simplifying the Screening Workflow for Extractables Testing
How to design an analytical workflow to screen for extractables and leachables The safety of pharmaceuticals, cosmetics, and foodstuffs may be compromised by chemical compounds in the various types of packaging and food contact materials (FCMs) that are in direct contact with the consumer product. Due to continuously increasing global regulations, the characterization of extractables...
Mission Improbable: Rapid Total Sugar Reporting for Beer by Mass Detection
Lion, one of the largest food and beverage companies in Australia and New Zealand, wanted to monitor fermentation profiles to completion as the company introduced voluntary nutritional panels across its beer portfolio. See what they gained by changing from electrochemical to mass detection.
A CRO Leads the Way for Biotherapeutics Amid its Own ‘Field of Dreams’
“If You Build it, He Will Come” Urged on by a voice no one else could hear, Ray Kinsella, played by Kevin Costner in the movie Field of Dreams, cleared a few acres of corn to build a baseball field. Shortly after, magical things began to happen. When you first set eyes on the headquarters...
Analytical Tools for Developing Biosimilars: Part 3, Glycosylation, Aggregation, and Charge Variants
In biotherapeutics, the state of glycosylation has a direct and pronounced effect of the structure, stability, serum half-life, immunogeneicity and bioactivity of the molecule, and constitutes a critical quality attribute (CQA). In our study, characterizing and comparing the glycosylation profile of infliximab began by examining the glycosylation profiles from intact and reduced subunit mass data.
Analytical Tools for Developing Biosimilars: Part 2, Peptide Analysis
Advancements in High-Resolution Analytics for Characterization of Innovator and Biosimilar Therapeutics Part 2: Peptide Analysis of Infliximab
How Analytical Technologies Support the Development of Biosimilar Drugs
Advancements in High-Resolution Analytics for the Characterization of Innovator and Biosimilar Therapeutics As the pharmaceutical industry continues to evolve its focus from small-molecule drugs to balanced product portfolios that include protein therapeutics, analytical chemists are increasingly challenged to produce routine and automated characterization workflows that move innovator and biosimilar biopharmaceutical products forward through development and...
Popular Topics
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (23) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (20) mass detection (16) mass spectrometry (MS) (54) method development (13) STEM (12) sustainability (12)