-
Charge Detection Mass Spectrometry (CDMS): Unprecedented Direct Measurement for the Characterization of Mega-Mass Biomolecules
Whether you are analyzing adeno-associated viruses (AAVs) or virus-like particles, nucleic acids such as RNAs or lipid nanoparticles (LNPs), proteins, or protein complexes, simple and fit-for-purpose tools with speed, accuracy, and precision are essential for driving biotherapeutic innovations forward. In recent years, scientists have increasingly turned to charge detection mass spectrometry (CDMS).

Recent Posts
Stronger Together Against COVID-19
At Waters, we have a long history of working alongside the scientific community in times of need through collaboration. Today, as the world continues to battle COVID-19, that collaborative approach is more important than ever. Ultimately, this challenge is far too great for any organization to tackle alone. That is why we created the Waters COVID-19 Innovation Response Team. In the early...
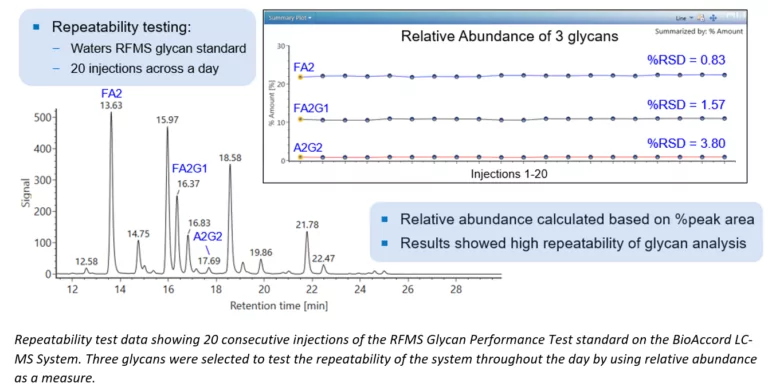
Waters GlycoWorks RapiFluor-MS N-Glycan Kit Improves Reproducibility and Speed of N-Glycan Analysis Through Innovation and Automation
Glycosylation is an important post-translational modification that can impact the safety and efficacy of protein therapeutics and is therefore one of the most analyzed product attributes during development and quality control. In released N-glycan analysis, glycans are cleaved from proteins via enzymatic digestion and are chemically derivatized for relative quantification by liquid chromatography coupled to fluorescence and mass spectrometry, or capillary electrophoresis with laser-induced fluorescence detection. The inherent challenges of N-glycan analysis have pushed the industry to improve upon...
Serving Our Customers Through a Pandemic
Early on in the pandemic, Adam Beard, Waters’ Vice President of Global Services, wrote about how our employees were “Delivering Benefit While Supporting the Front Lines of a Global Pandemic” in a post on LinkedIn. Today, that critical work continues. Recently, we spoke to some of our colleagues from our global service and manufacturing teams that...
The Impact of Complex Global Supply on the Nitrosamine Crisis
When a crisis occurs, hindsight is a great teacher. Any company developing or manufacturing products that impact human health can learn from the recent nitrosamine impurity contamination of angiotensin II receptor blockers (ARBs) by reviewing their own quality processes for risks that could result in a similar contamination issue. Analytical data should assure confidence in...
Choosing the Correct Column for Chromatographic Selectivity
When developing a method for the routine multiresidue analysis of pesticides in food commodities there are several factors that need to be considered when selecting which liquid chromatography (LC) column to use. The requirements include: Good retention for a range of physiochemical properties (polar, non-polar, base, acid). Moderate to high resolution (peak capacity). Sample extracts...
Change Up Your Routine (Analysis)
Enhancing performance, mitigating risk, and improving efficiency Today, Waters introduced the Arc HPLC, a modern liquid chromatography system that replicates established test methods while delivering improved performance. HPLC has been a cornerstone of routine analytical testing for decades. It has proven to be particularly important for quality control and manufacturing support laboratories in the pharmaceutical...
Popular Topics
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (22) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (20) mass detection (16) mass spectrometry (MS) (55) method development (13) STEM (12) sustainability (12)