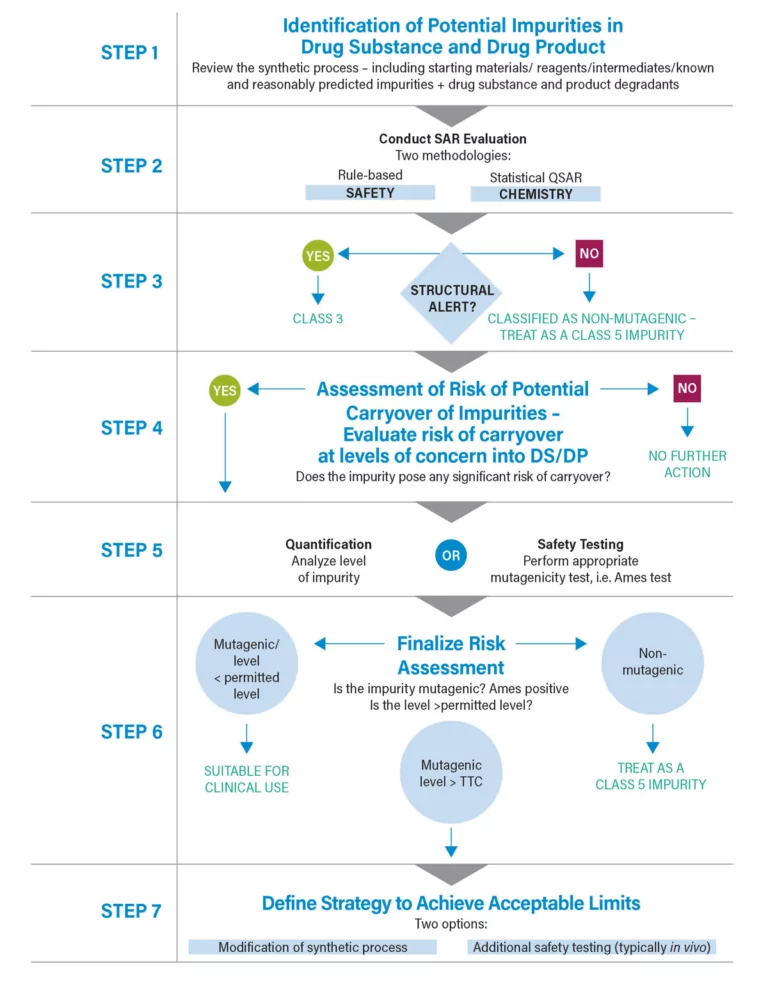
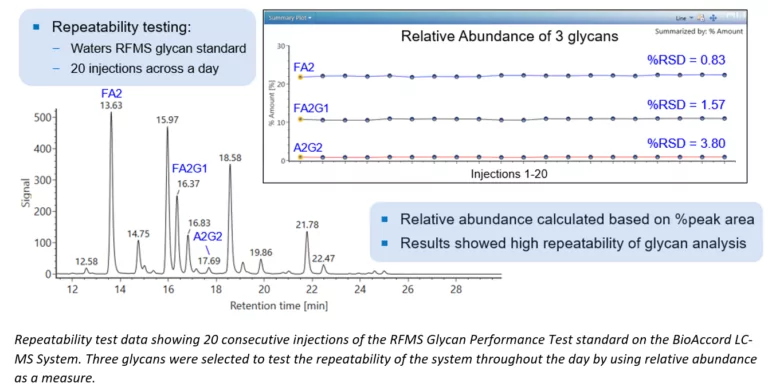
The Evolving Regulations Guiding Nitrosamines
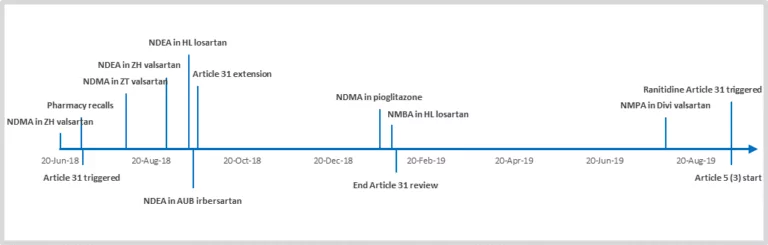
In July 2018, the European Medicines Agency (EMA) reported the recall of a number of products containing the active pharmaceutical ingredient (API) valsartan due to contamination with a known carcinogen, dimethyl nitrosamine, also referred to as N-nitroso dimethylamine (NDMA). This action set off a chain of events that led to similar notifications of recalls for…