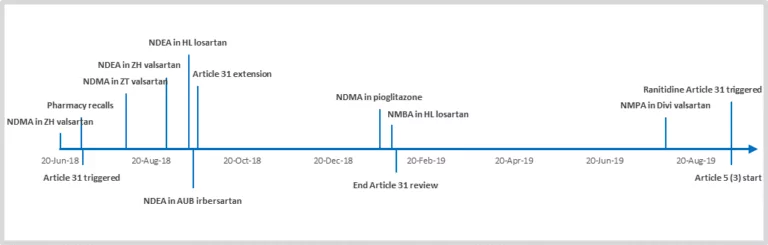
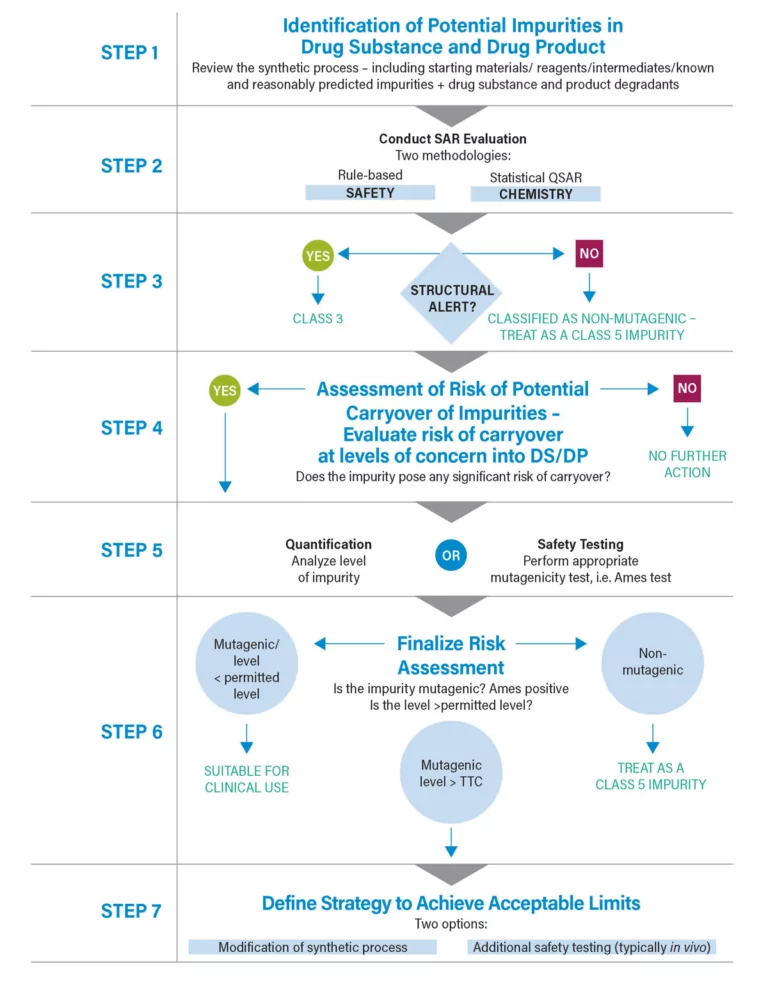
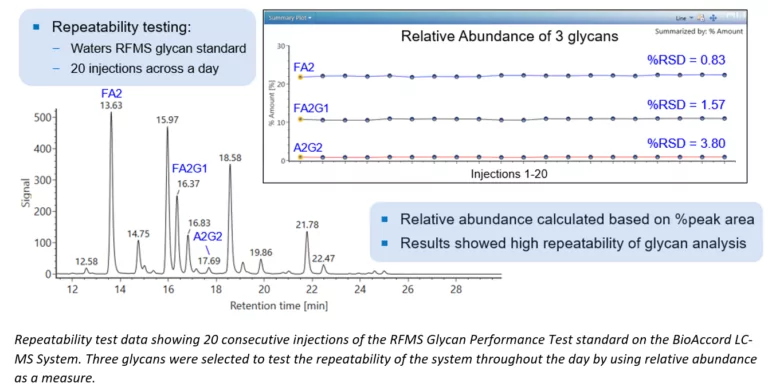
Boosting Biopharma Productivity with Routine LC-MS
As scientific advances continue to carry researchers into new arenas, it is vital that technology evolves in tandem. At Waters, we understand that it is only by leveraging fit-for-purpose technologies that researchers are able to unleash their creativity and make pioneering scientific breakthroughs. Scientists working within the rapidly growing biopharmaceutical industry are no exception. With…