-
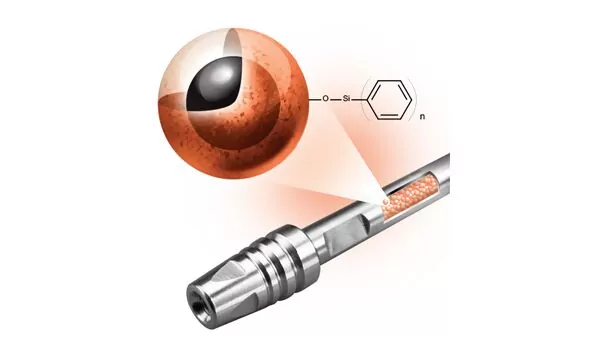
OligoWorks SPE Kits: Boosting Oligonucleotide Bioanalysis Efficiency
New OligoWorks Solid Phase Extraction (SPE) Kits for extraction and bioanalysis of oligonucleotides deliver advantages over liquid-liquid extraction (LLE) and other existing SPE kits and sample preparation methods. Read on to discover how.
Recent Posts
How to improve analytical method transfers for biotherapeutics
“Assuming continuous improvement is a worthy goal, there is every reason to improve validated chromatographic methods if you’re working with the right instrument technology,” says Eric Grumbach of Waters. See what that means for developing and transferring methods for biologic drugs.
Setting a New Standard for Reserved-Phase Separations of Monoclonal Antibodies and Antibody Drug Conjugates
A new approach to stationary phase chemistry for reversed-phase LC analysis of proteins, including monoclonal antibodies and antibody drug conjugates, delivers higher fidelity data especially for MS-based peak identification.
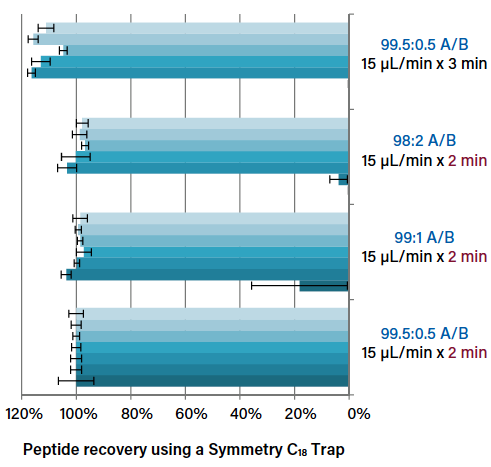
Columns Matter, Too: Choosing Optimal Conditions for Trap-and-Elute Nanoflow and Microflow LC-MS
Considerations for optimizing trapping conditions, such as flow rate and trapping volume, in nanoscale and microflow LC-MS.
UPLC Chromatography Gets an Update: Because Science Never Quits
We've updated our UPLC systems to enable you to find faster, simpler, and better ways to do your work. Take a closer look at our new and improved ACQUITY UPLC PLUS Series.
What is Changing Analytical Method Transfer Today? Part 3: Methods Across Borders
One scientist at a major global pharma company estimated that an analytical method could see up to 100 transfers in its lifetime. Increasingly, such transfers will take place across geographic borders. How ready are you to mobilize your methods?
From the Results of Yesterday to the Biologic Drugs of Tomorrow
A thousand small delays and opportunities for error can snowball over the years of a complex biotherapeutic drug development program. They add up to lost time and increased risk in an endeavor that has little tolerance for either. It doesn’t have to be this way.
Popular Topics
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (22) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (20) mass detection (16) mass spectrometry (MS) (54) method development (13) STEM (12) sustainability (12)