Slalom Chromatography: A High-Speed Path to Nucleic Acid Separation

Slalom chromatography is no longer just a scientific curiosity, it’s a comeback story. Once sidelined due to limited reproducibility and unclear mechanisms, this technique is now gaining momentum thanks to the rise of nucleic acid therapeutics and advances in UHPLC systems. But its revival wasn’t accidental. It took a year-long deep dive into DNA physics, a dose of scientific curiosity, and a commitment to solving real-world challenges in cell and gene therapy, including improving mRNA production processes at scale.
A Technique Decades in the Making
First observed in the 1980s by researchers like Barry Boyes and Kenichi Kasai, slalom chromatography intrigued scientists with its reversed elution behavior, like longer DNA fragments eluting later than shorter ones. The concept was visually compelling, likened to DNA molecules weaving through packed particles like skiers navigating slalom gates. Yet, without a clear mechanistic understanding, the technique faded into obscurity.
Fast forward to 2022, when Waters scientists Fabrice Gritti, Matthew Lauber, and Kevin Wyndham asked a bold question: could slalom chromatography be brought back from the dead? Gritti’s journey into the physics of DNA stretching under shear forces laid the groundwork for a new generation of slalom columns. His research revealed that double-stranded DNA and RNA undergo entropic elastic stretching, uncoiling without breaking bonds, while single-stranded molecules do not. This insight opened the door to separating dsRNA impurities from mRNA vaccine products, addressing a critical need in modern biopharma.

Where Slalom Chromatography Excels
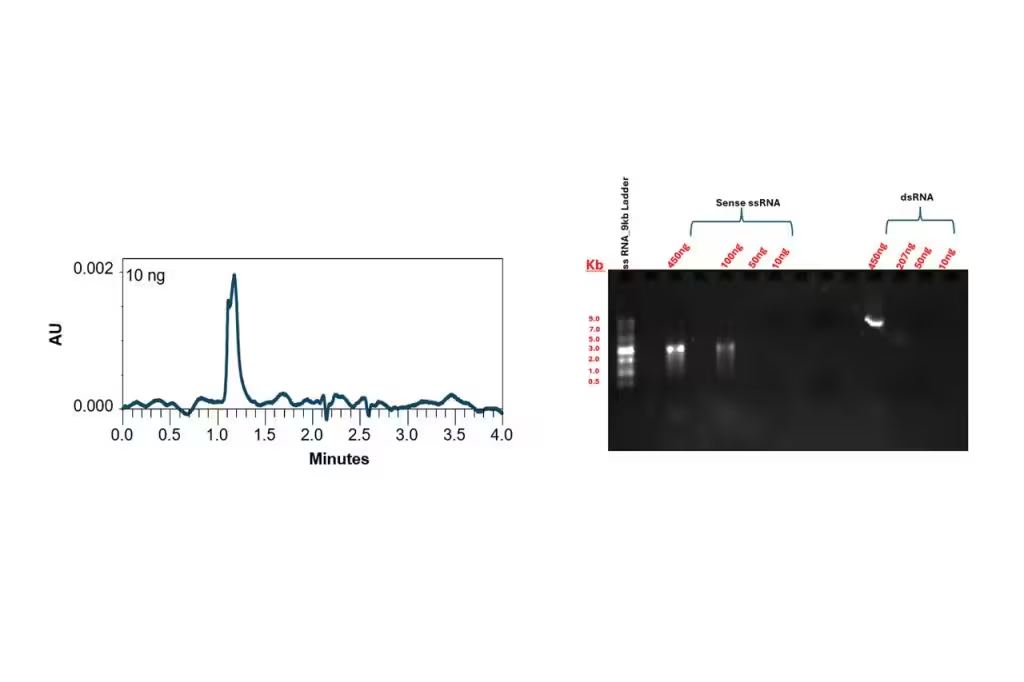
Slalom chromatography is transforming nucleic acid workflows with fast, reproducible separation and minimal sample prep. It’s particularly effective in applications such as DNA restriction mapping, plasmid topology analysis, and PCR product verification. Researchers can now isolate pure DNA bands, up to 1 µg, without relying on gels, and detect immunogenic dsRNA contaminants in mRNA production and large-scale mRNA products with greater confidence.
Its simplicity, often requiring just buffer exchange and dilution, makes it a low-risk, high-reward technique, especially for double-stranded nucleic acids. For many labs, it represents an efficient alternative to DNA gel electrophoresis or DNA ladder gel electrophoresis. With slalom chromatography, you can bypass questions like ‘How does gel electrophoresis separate DNA fragments?’ or ‘How do I interpret DNA data from gel electrophoresis?’, because it offers a more streamlined approach to analyzing double-stranded nucleic acids. Slalom chromatography gives your lab sharp resolution and fast fraction collection without the prep, smears, or guesswork of gels.
The Science Behind the Separation
At the heart of slalom chromatography is a unique separation mechanism. Under high-pressure flow, nucleic acid molecules stretch and longer strands interact more with the stationary phase causing them to elute later. This behavior flips the script compared to traditional size-exclusion chromatography (SEC).
Double-stranded DNA and RNA behave like flexible polymers, making them ideal candidates for slalom separation. In contrast, single-stranded and circular DNA do not stretch in the same way, limiting their interaction with the column. The technique is most effective for molecules in the 3–40 kilobase pair range, offering high-resolution separation based on molecular flexibility and length, critical for characterizing large biopolymers in cell and gene therapy.
Getting the System Right
To unlock the full potential of slalom chromatography, your system must be built for performance. UHPLC systems capable of pressures ≥10,000 psi are essential. Elevated temperatures help reduce viscosity and improve reproducibility, while bioinert materials prevent sample loss due to nonspecific adsorption. Standard UV detection at 260 nm works well, though multi-angle light scattering (MALS) can provide additional insights into molecular weight and size. For those looking to purify samples, a fraction collector can be a valuable addition.
Method Development: Precision Matters
Developing a robust slalom method requires attention to detail. Buffered mobile phases like tris-acetate-EDTA (TAE) are recommended, and maintaining elevated column temperatures ensures stability. Flow rate and ionic strength should be optimized within pressure limits to achieve sharp selectivity. Periodic column regeneration using low volumes of 1M spermidine helps remove impurities and extend column life.
Skipping temperature control or under-pressurizing the system can compromise resolution and reproducibility, so thoughtful setup is key.
From Theory to Impact
Waters became the first company to commercialize a slalom chromatography column, marking a milestone in separation science. But the journey doesn’t end here. As customers begin to share feedback and explore new applications, the technique will continue to evolve, driven by curiosity, collaboration, and a willingness to think outside the box.
Whether you’re verifying PCR products or purifying plasmids, slalom chromatography offers a fast, reliable, and reproducible way to separate double-stranded nucleic acids. It’s not just a technique. It’s a testament to the power of interdisciplinary thinking and scientific perseverance.
Ready to explore what’s possible with slalom chromatography? Take a deeper dive with these resources:
Popular Topics
ACQUITY QDa (17) bioanalysis (12) biologics (15) biopharma (29) biopharmaceutical (38) biosimilars (12) biotherapeutics (20) case study (18) chromatography (14) data integrity (23) food analysis (12) HPLC (15) LC-MS (25) liquid chromatography (LC) (24) mass detection (16) mass spectrometry (MS) (58) method development (13) sustainability (12)