Data Integrity Matters | Limiting Access to Tools That Could Be used to Manipulate Data (Part 3)
How do data integration and reintegration affect data integrity?
Our expertise drives innovation, development, and application of informatics and software that continue to enable leading pharmaceutical, environmental, food & beverage and chemical materials organizations to accelerate decision-making, improve laboratory effectiveness, and get products to market faster.
How do data integration and reintegration affect data integrity?

Learn how the ACQUITY QDa Mass Detector brings the power of mass detection for purification to Medicilon, helping this CRO meet the growing demand for outsourced services in the pharmaceutical and biotechnology industries.

Scientists from Waters and Pfizer collaborated to take on the challenge of engineering a novel reversed-phase LC column for high-throughput separations with enhanced resolution, selectivity, and recovery of target mAbs and ADCs.
What procedures can an analyst use to ensure that a chromatography system is ready to begin analyzing samples, without the fear that the system suitability tests would immediately fail?

Halting a chromatographic analysis where the system is not performing correctly or when another error is evident in the separation is a valuable and scientific way to avoid creating unusable data that needs to be subsequently invalidated following a defined analytical lab error/result SOP. But what do you do with that orphan data?
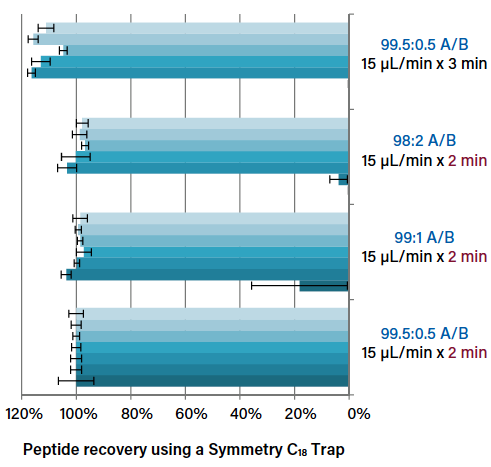
Considerations for optimizing trapping conditions, such as flow rate and trapping volume, in nanoscale and microflow LC-MS.
We’ve updated our UPLC systems to enable you to find faster, simpler, and better ways to do your work. Take a closer look at our new and improved ACQUITY UPLC PLUS Series.

One scientist at a major global pharma company estimated that an analytical method could see up to 100 transfers in its lifetime. Increasingly, such transfers will take place across geographic borders. How ready are you to mobilize your methods?
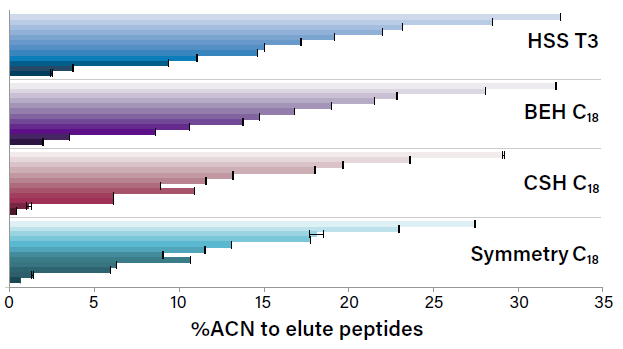
On the impact of retentivity and characteristics of the stationary phases for reversed-phase proteomic separations and how you can use them to your advantage.
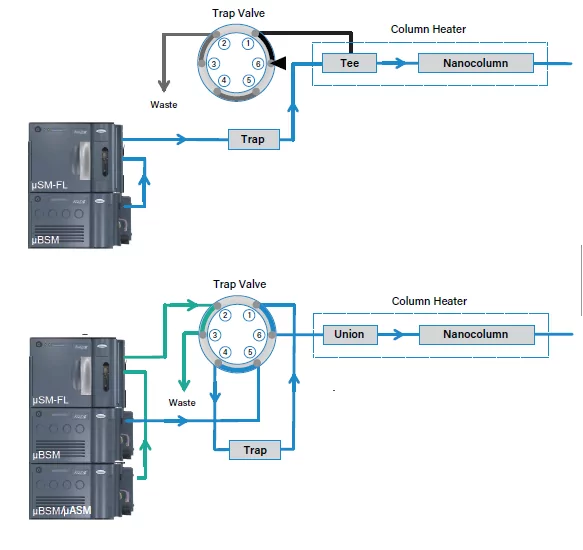
Can pairing column stationary phases improve nanoflow and microflow LC-MS results? Let’s take a closer look at the basics of trapping and the advantages and considerations for implementing a trapping step in a LC separation in part 1 of this 3-part series.

Is your analytical method bound for the lab down the hall, or the lab halfway around the world? If you’re an analytical or QC scientist, get ready for more method transfers between organizations. We explore the complexities of analytical method transfer in part 2 of our ongoing series.

There’s a new analytical testing landscape taking shape, and it’s having a big effect on how we develop, transfer, and update methods. Data quality and business efficiency are both at stake. Are you ready?
ACQUITY QDa (17) bioanalysis (11) biologics (14) biopharma (26) biopharmaceutical (36) biotherapeutics (17) case study (17) chromatography (14) data integrity (22) food analysis (12) HPLC (15) LC-MS (22) liquid chromatography (LC) (20) mass detection (16) mass spectrometry (MS) (55) method development (13) STEM (12) sustainability (12)