
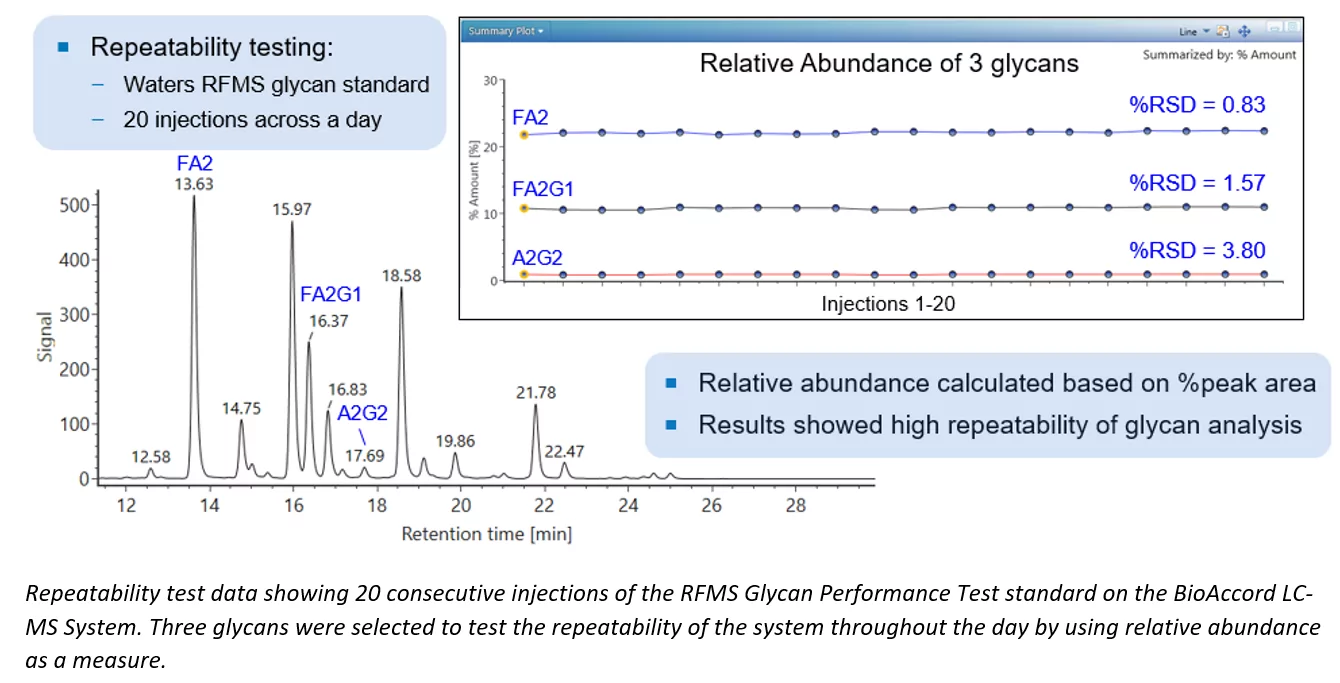
Glycosylation is an important post-translational modification that can impact the safety and efficacy of protein therapeutics and is therefore one of the most analyzed product attributes during development and quality control. In released N-glycan analysis, glycans are cleaved from proteins via enzymatic digestion and are chemically derivatized for relative quantification by liquid chromatography coupled to fluorescence and mass spectrometry, or capillary electrophoresis with laser-induced fluorescence detection. The inherent challenges of N-glycan analysis have pushed the industry to improve upon…