Medical devices play a crucial role in the treatment, diagnosis, and care of patients, and medical device manufacturers must rigorously test the chemical structure and physical property materials to ensure both the devices and their packaging meet regulatory standards and performance criteria, guaranteeing their suitability for intended use.
Waters and TA Instruments combined instruments, software, consumables, and support enables robust workflows to streamline analytical testing and materials measurements – whether your lab is focused on chemical and materials property testing, extractables and leachables (E&L) studies, or durability assessment. Leveraging cutting-edge technological advancements and strategic partnerships with Waters, you can accelerate innovation and commercialization processes, simultaneously minimizing risks, cutting costs, and ensuring utmost patient safety.
Waters software streamlines data acquisition, processing, and reporting medical device materials analysis results.
Perform structural elucidation of unknown compounds above the analytical evaluation threshold in less time with waters_connect Software Solutions, offering a simple workflow that includes scientific library creation, multivariate statistical analysis, and reporting.
Our full range of columns and consumables can be easily integrated into your chromatography and mass spec workflows.
Discover increases in LC efficiency, resolution, sensitivity, accuracy, speed, and reliability with ACQUITY sub-2-μm UPLC particle columns for your medical device materials analyses.
Achieve accurate results for your medical device materials analyses using streamlined Waters instrument services.
Meet your lab’s evolving needs with Waters extensive portfolio of LC and LC-MS instrument support, qualification and services that offer quality, knowledge, and reliability for optimum lab productivity.
Take advantage of the most advanced analytical technology available with innovative payment solutions from Waters Capital and bundle your complete laboratory solution in one easy monthly payment.
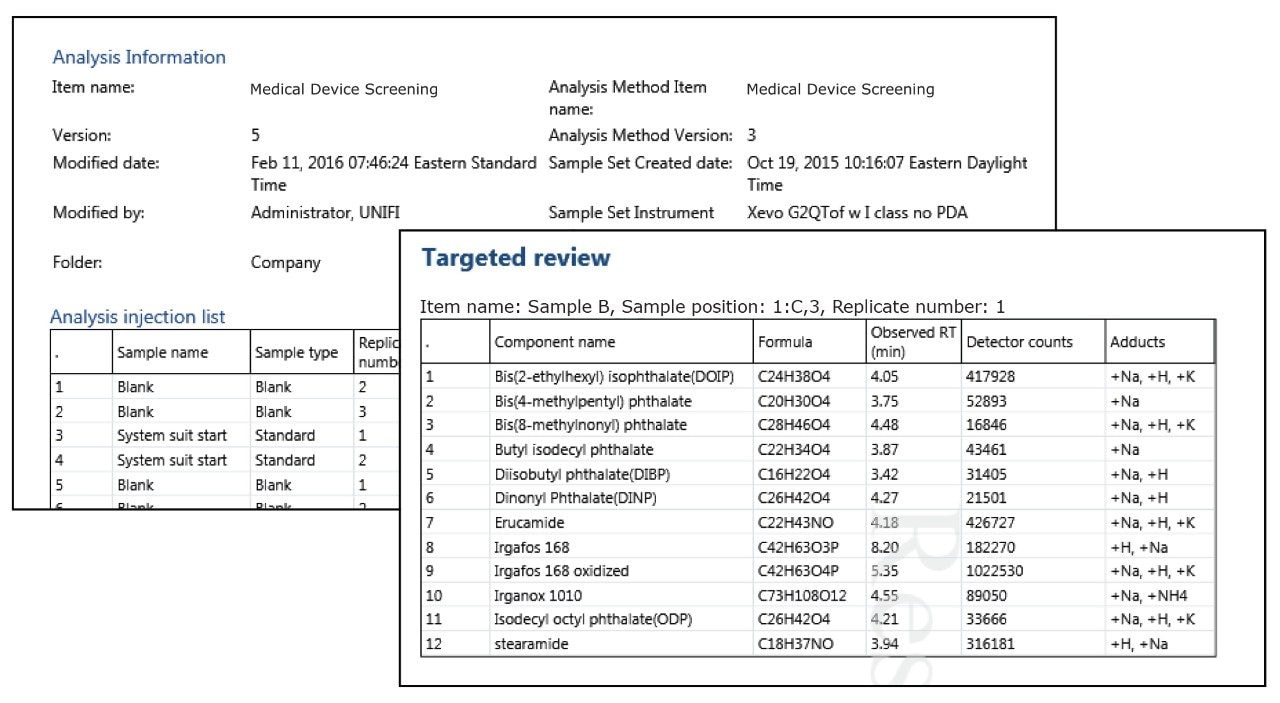
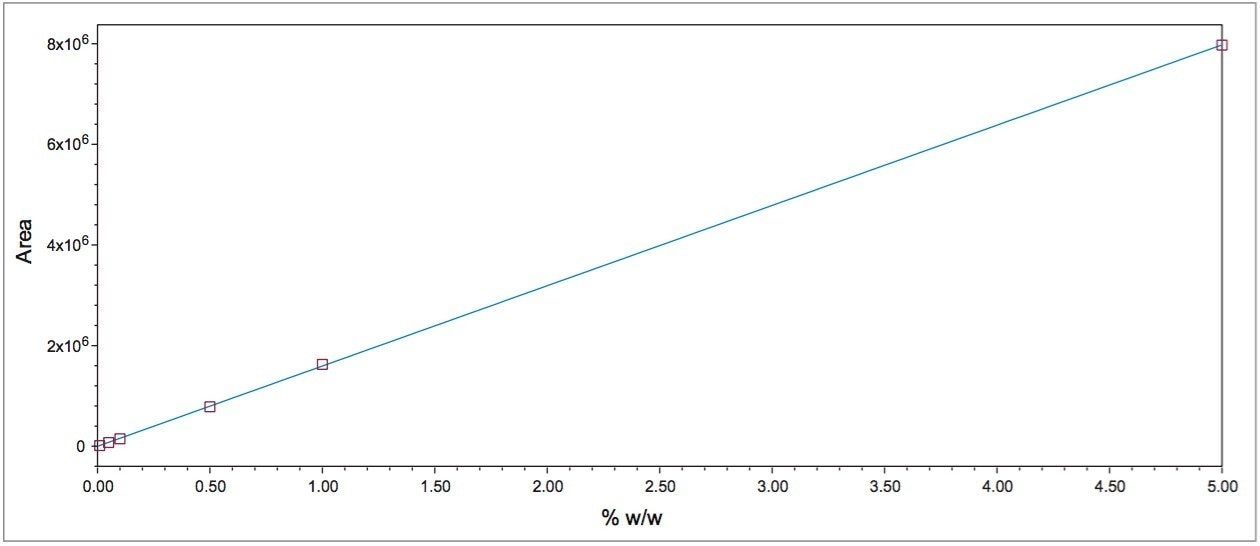
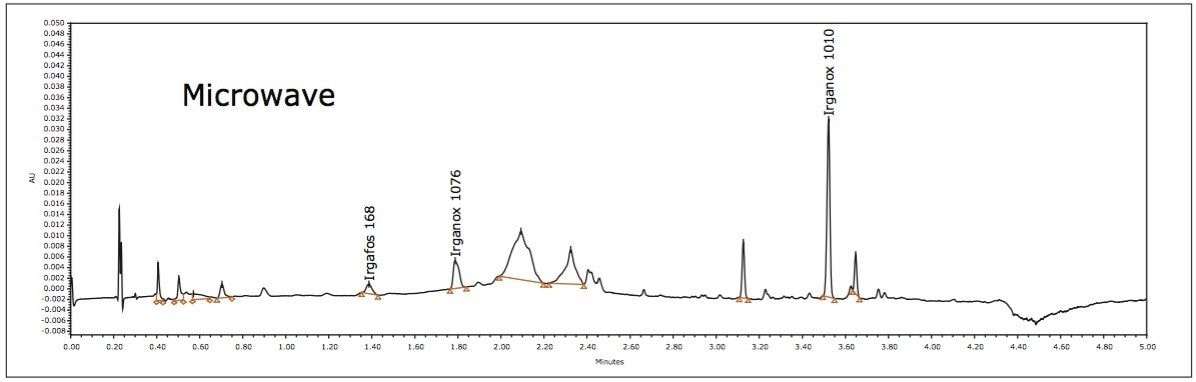
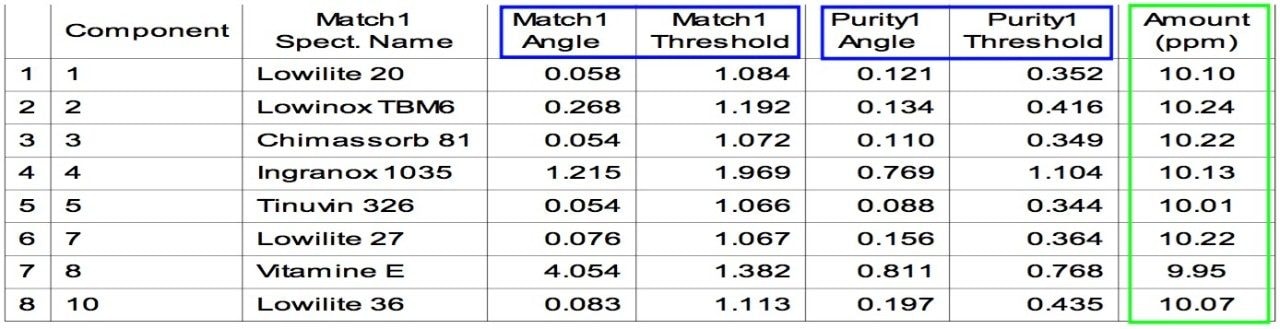
Medical devices must meet strict global standards to ensure safety, quality, and performance. The materials used such as polymers, metals, and coatings require thorough testing to verify their chemical composition, durability, and biocompatibility. Even minor impurities or residues can affect device reliability and patient safety. Compliance with regulations like ISO 10993, FDA 21 CFR Part 11, and EU MDR is critical for successful market approval.
Waters offers advanced materials testing solutions tailored for the medical device industry. Our analytical technologies including liquid chromatography, mass spectrometry, and infrared spectroscopy help manufacturers detect contaminants, monitor material degradation, and ensure consistent product quality. These solutions support extractables and leachables studies, failure analysis, and raw material selection to enhance device performance and lifespan.
By streamlining workflows and improving data accuracy, Waters helps manufacturers reduce testing time and costs while meeting regulatory requirements. With our expertise, companies can confidently navigate complex compliance landscapes and deliver safe, innovative medical devices to market faster.