
Reversed-phase HPLC methods development can take anywhere from weeks to months, incurring large operational cost. By utilizing UltraPerformance LC (UPLC) Technology for methods development, a 6-fold improvement in throughput can be realized. This, in turn, reduces cost per sample and time of analysis considerably while maintaining or improving separation integrity. By developing rapid, high resolution analytical methods, products can be brought to market faster, therefore, improving the overall profitability of the assay. Here we demonstrate this methods development approach in the separation of paroxetine hydrochloride and its related compounds.
Reversed-phase HPLC methods development can take anywhere from weeks to months, incurring large operational cost. By utilizing UltraPerformance LC (UPLC) Technology for methods development, a 6-fold improvement in throughput can be realized. This, in turn, reduces cost per sample and time of analysis considerably while maintaining or improving separation integrity. By developing rapid, high resolution analytical methods, products can be brought to market faster, therefore, improving the overall profitability of the assay.
A new method can be developed efficiently if experimental design is well thought out. Common methods development approaches include: conducting a literature search, trial and error, a step-wise iterative approach or a systematic screening protocol. A systematic screening protocol that explores selectivity factors such as pH, organic modifier, and column chemistry will be the premise of this strategy. This approach allows chromatographers to quickly determine which experimental parameters are most effective in manipulating the selectivity of a separation. By employing this strategy, the total number of steps necessary to develop a method are reduced, therefore, providing an efficient and cost effective approach.
In this application note, combinations of selectivity factors (pH, column chemistry, and organic modifier) in UPLC separations were examined to develop high resolution chromatographic methods. Once the best combination of factors was selected, gradient slope and temperature were optimized. This methods development approach is demonstrated by developing a separation for paroxetine hydrochloride and its related compounds.
As depicted in Figure 1, a result matrix of 14 chromatograms is generated by evaluating three Bridged Ethylene Hybrid (BEH) columns at low and high pH and a silica (HSS) column at low pH, with two different organic modifiers. Each experimental result was evaluated for retentivity, peak shape, and resolution.
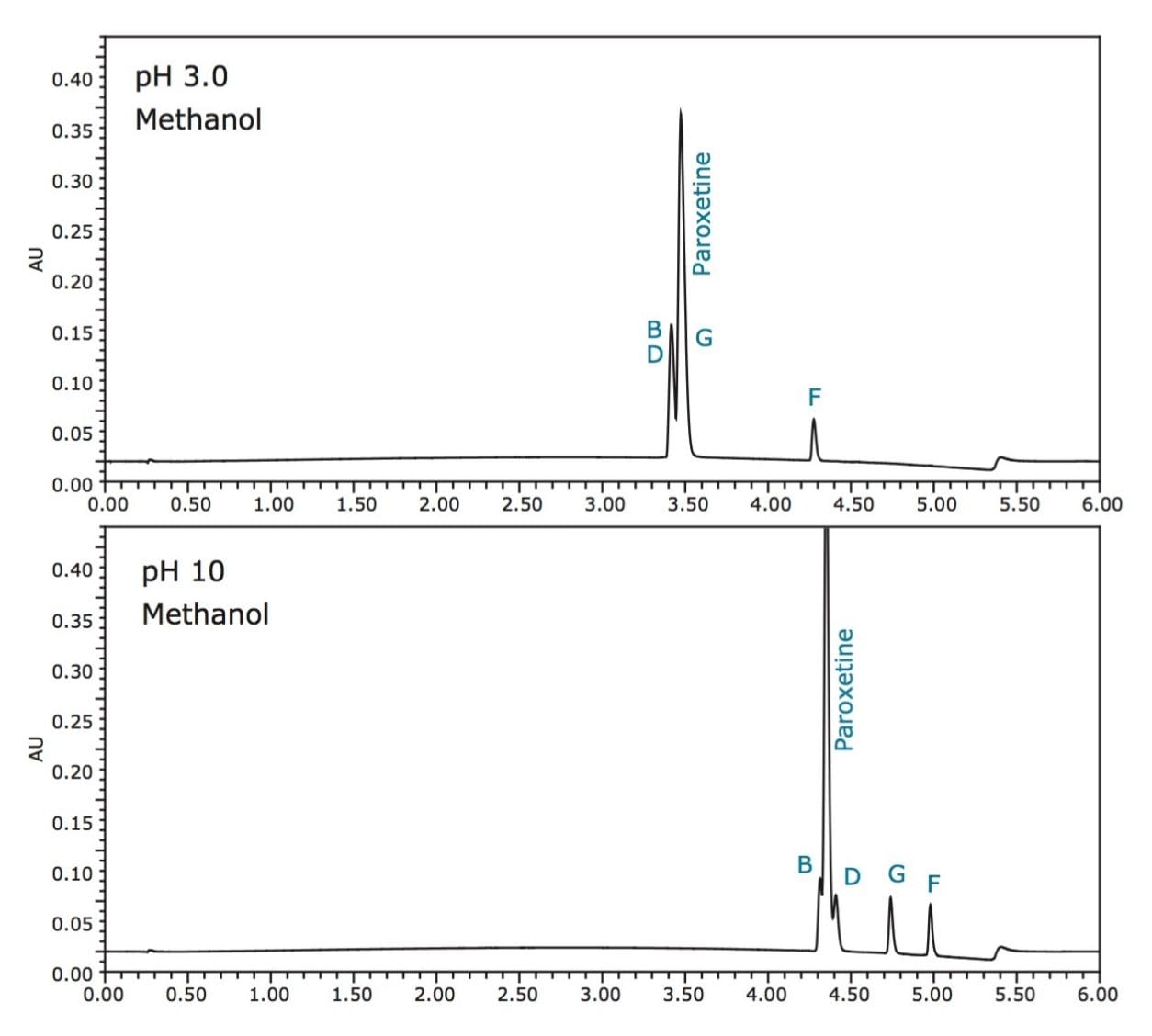
By first evaluating the data acquired at low and high pH, the retention characteristics, loadability, and overall resolution of the mixture of analytes can quickly be determined. Paroxetine is an alkaline species with a pKa of 9.8. It is, therefore, in its neutral charge state when the mobile phase is increased to pH 10. As seen in Figure 2, acidic mobile phase pH results in poor resolution of paroxetine and related compounds. Alkaline pH provides better retention and resolution of all components due to the neutral charged states of the analytes.
Once pH is selected, a comparison of different stationary phases is made. As shown in Figure 3, all three BEH columns show potential for resolving all components. The ACQUITY UPLC BEH C18 Column was selected to carry out the separation.
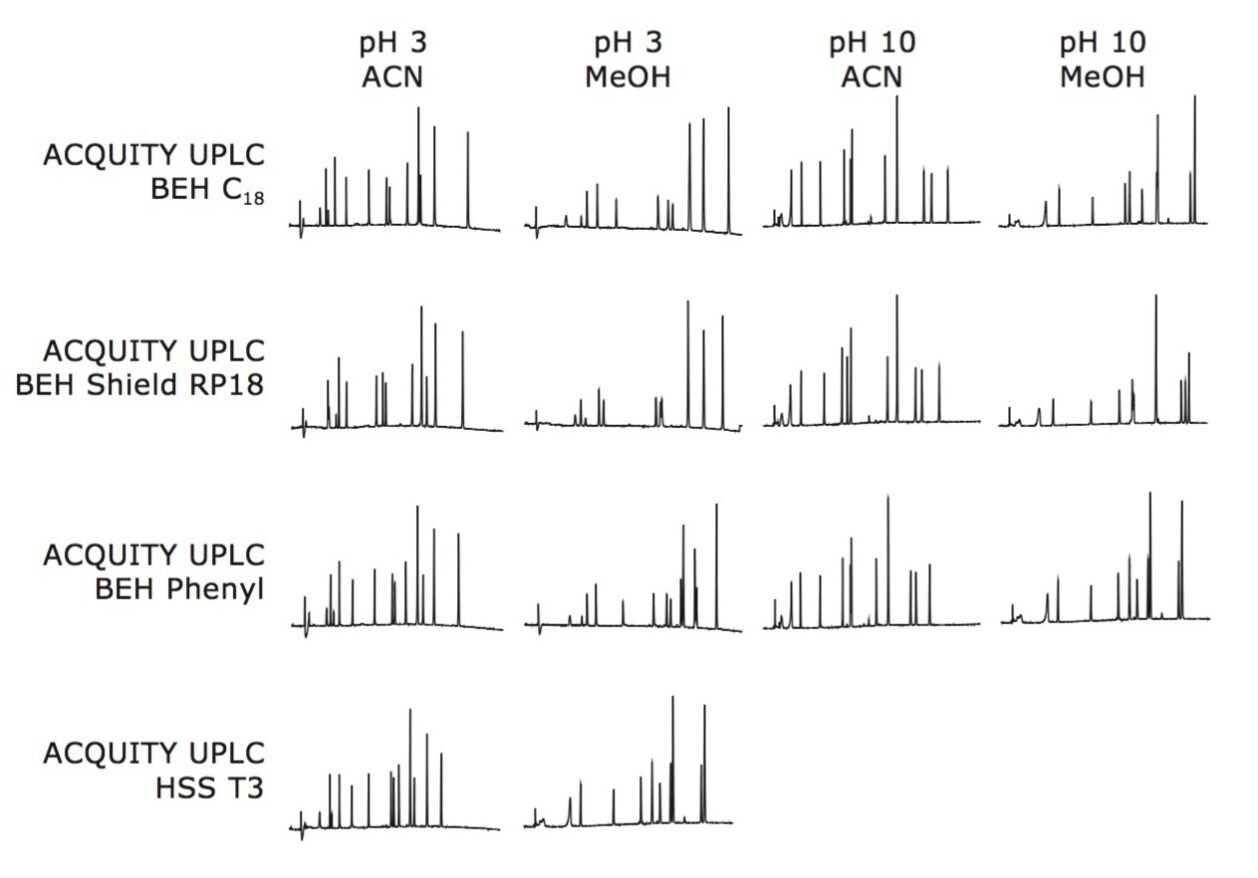
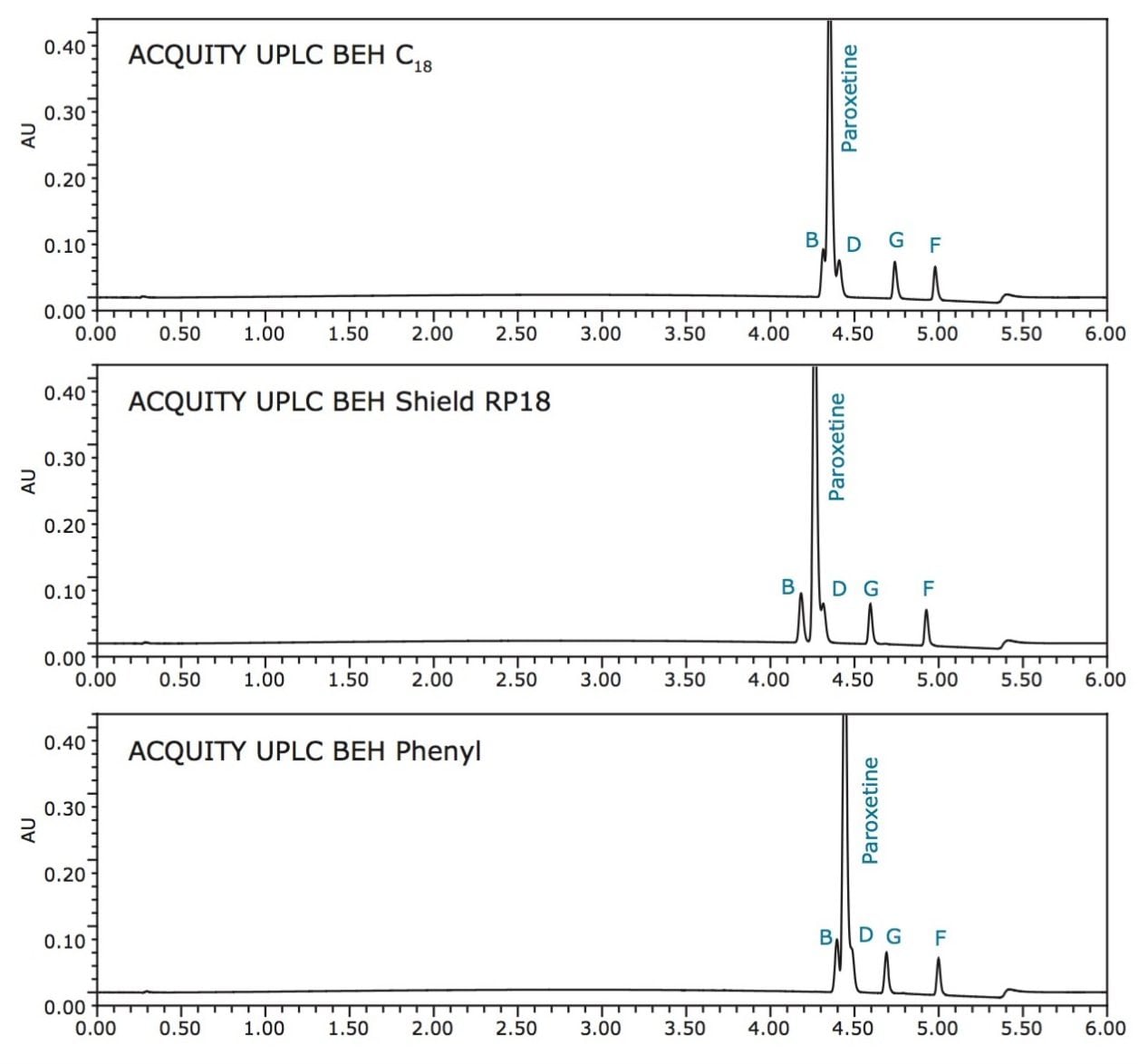
Lastly, the organic modifier is selected. Methanol offers a different selectivity than acetonitrile, and is a weaker elution solvent at equivalent concentration. This results in greater retention of the analytes. For this set of components, acetonitrile offers a better separation, as depicted in Figure 4.
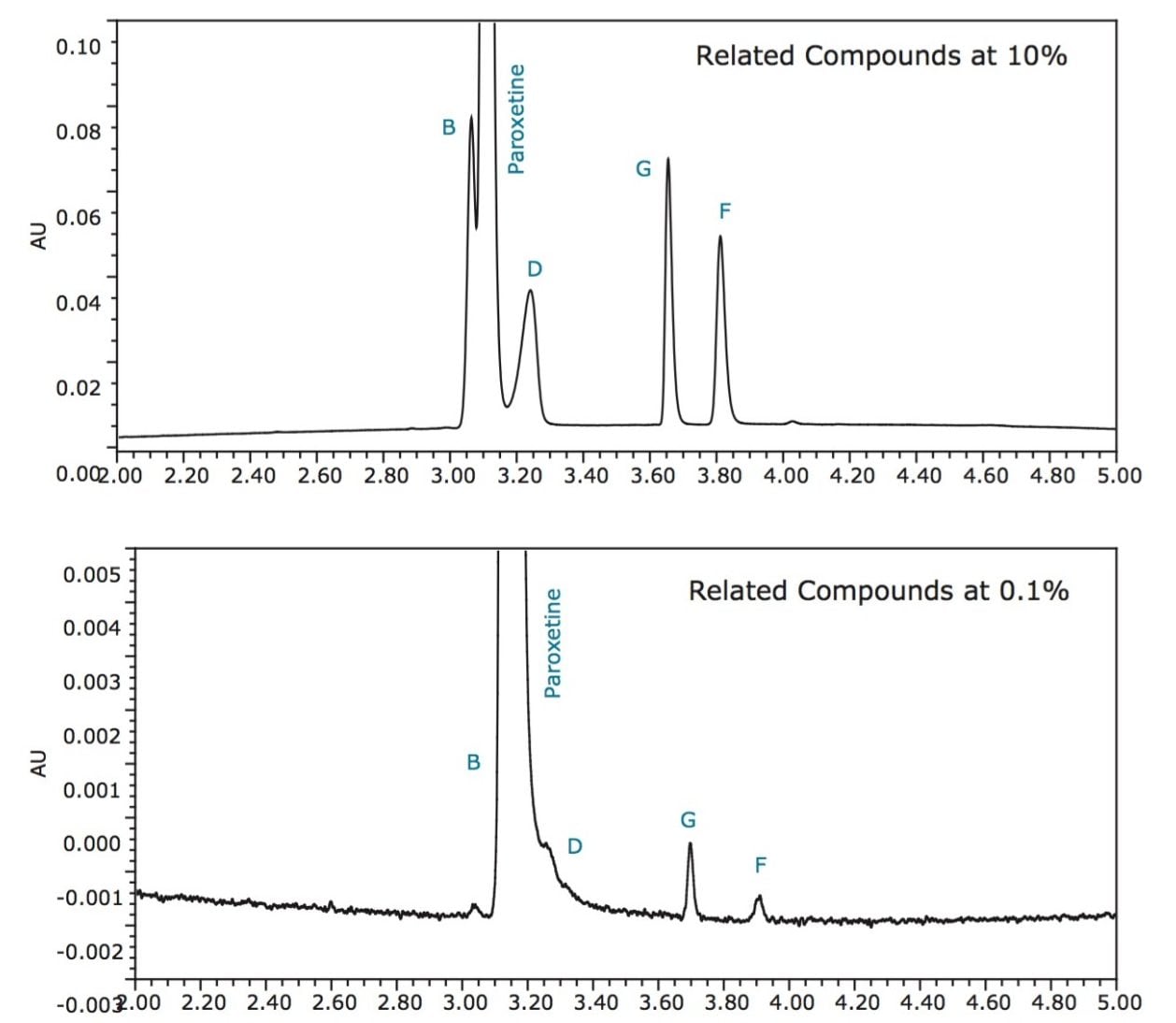
During our initial method screening, the related compounds were spiked into the solution at a 10% concentration level relative to paroxetine for ease of identification. For method optimization, the concentration of the related compounds was reduced from 10% of paroxetine to the target concentration of 0.1%, as shown in Figure 5. However, at the 0.1% concentration level, inadequate resolution among paroxetine and related compounds B and D resulted due to disparate levels of concentration making for a more challenging separation. In efforts to improve the separation, gradient slope and temperature were manipulated.
Changing gradient slope is often a balance between resolution and sensitivity. Although selectivity change can occur, most often a steeper gradient slope will result in a reduction in resolution and an increase in sensitivity, while a shallower gradient slope will result in an increase in resolution and a decrease in sensitivity.
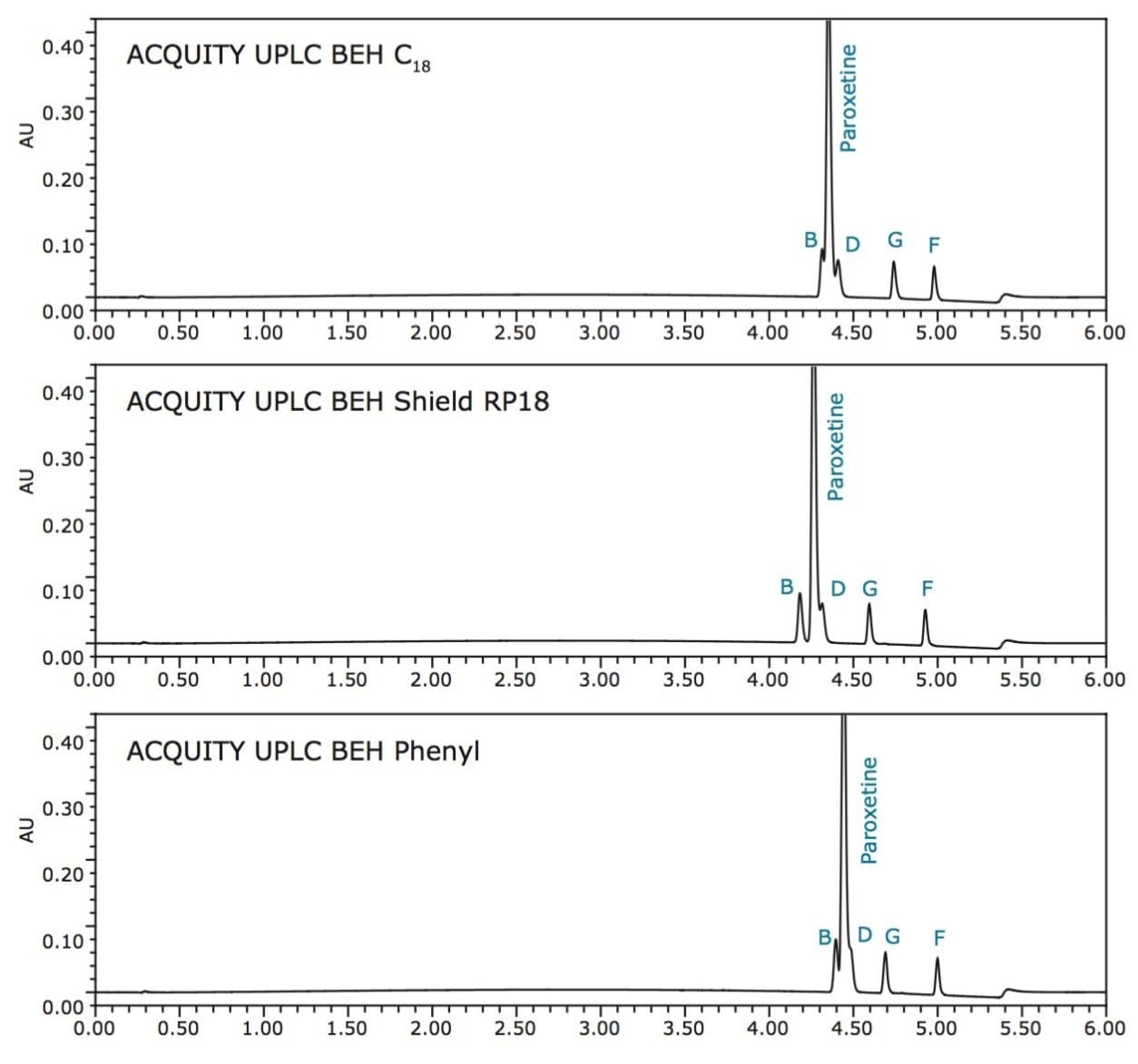
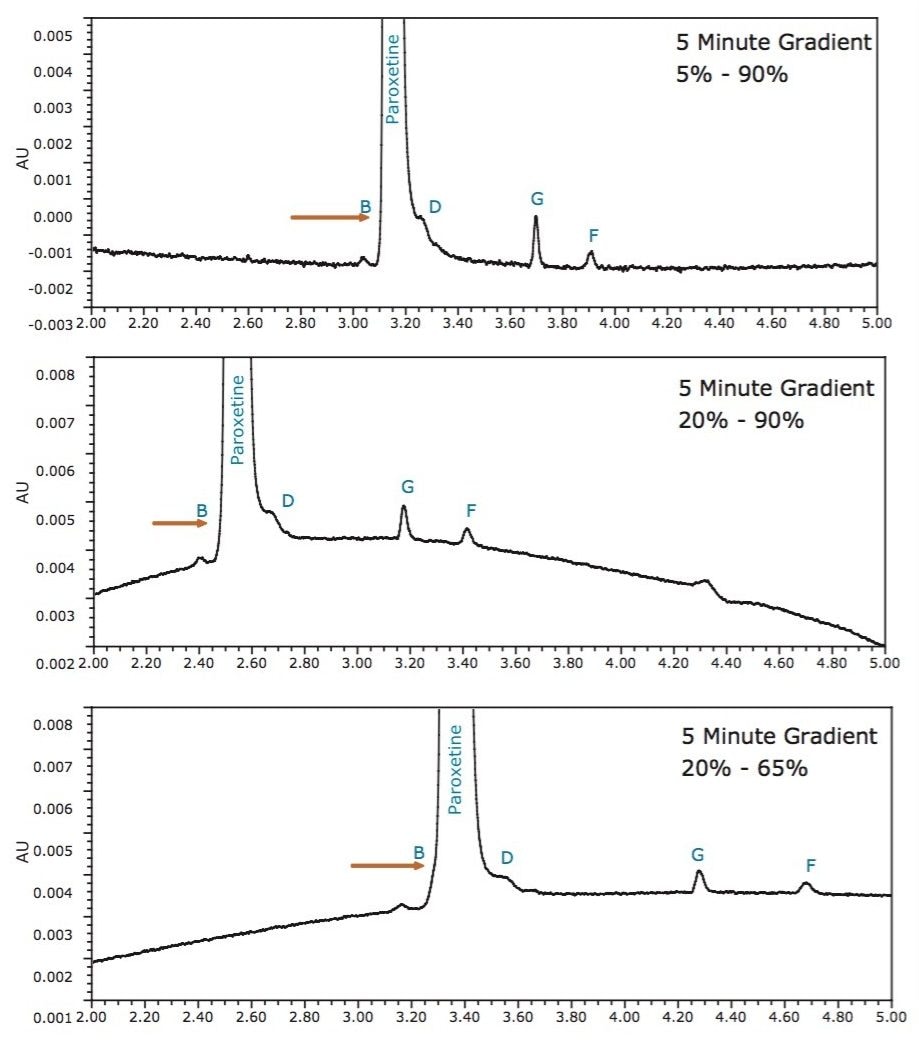
In efforts to improve resolution, the gradient slope was flattened by changing the % organic at the start and then endpoint of the gradient. In this case, marginal improvement was made by altering the gradient slope as depicted in Figure 6. Using the 20–65% acetonitrile gradient, the influence of column temperature was then explored.
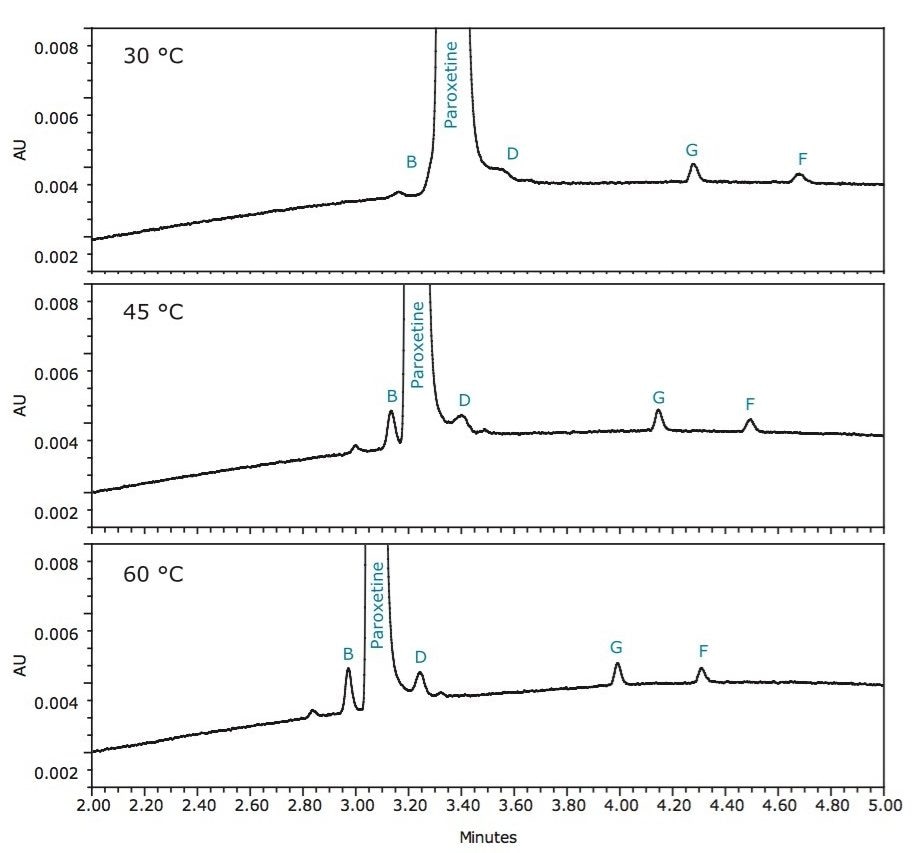
Temperature affects every chemical process that occurs. Analyte diffusivity, sample loadability, and peak shape dramatically improved with increasing temperature. At 60 °C, adequate separation of related compounds from paroxetine was achieved; therefore, no further optimization was necessary.
Separation was performed on an ACQUITY UPLC BEH C18, 1.7 µm, 2.1 × 50 mm Column at 60 °C. Mobile phase A contained 20.0 mM ammonium bicarbonate with 1.2% ammonium hydroxide. Mobile phase B was acetonitrile. A 5 minute gradient from 20 to 65% acetonitrile was performed. Flow rate was 0.5 mL/min.

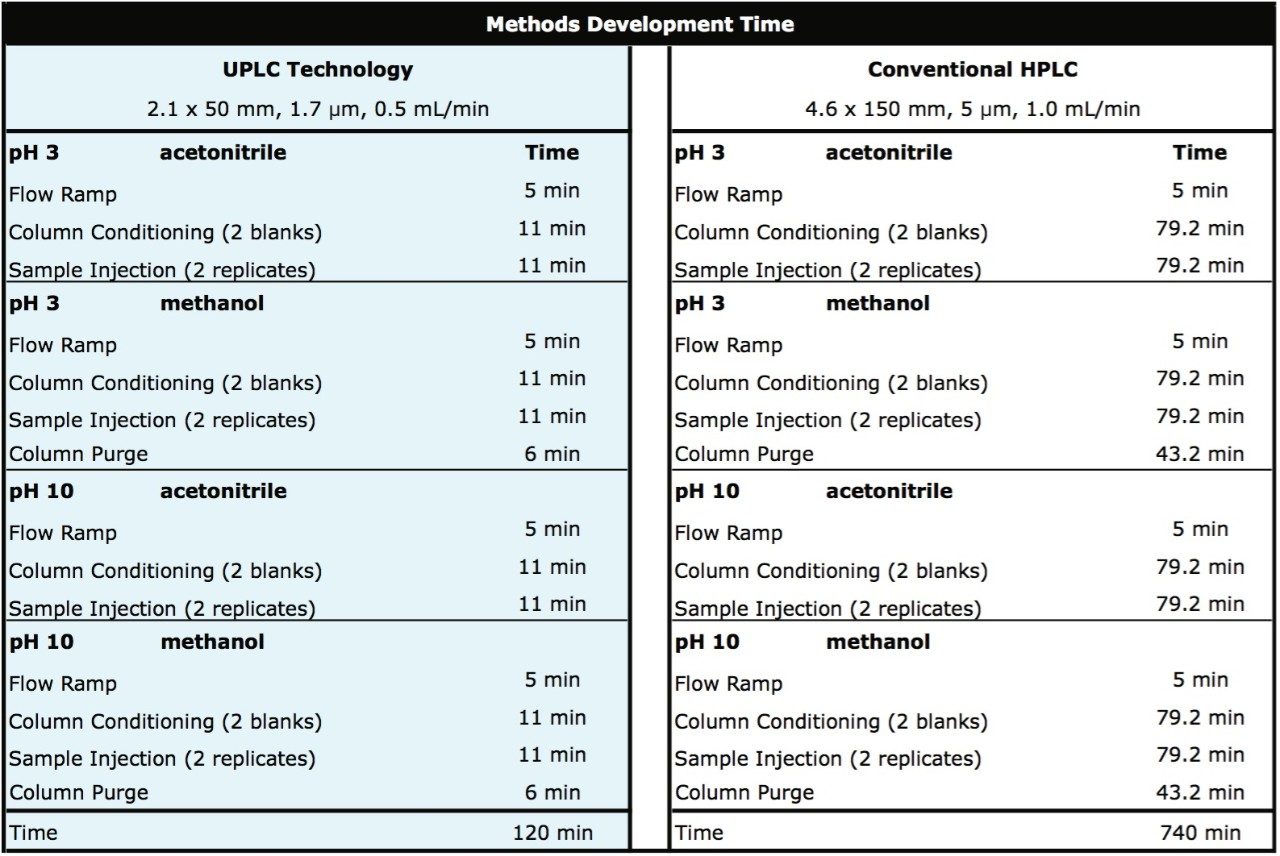
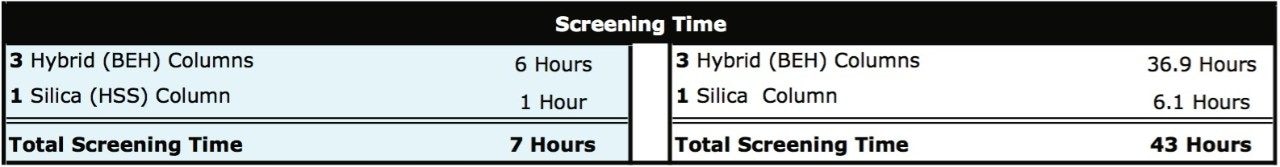
Productivity improvements associated with employing UPLC Technology for methods development are depicted below in Table 1. By comparing the UPLC methods development strategy outlined previously to one directly scaled to conventional HPLC, a 6-fold improvement in time is observed. This significantly reduces the overall instrument time required to develop chromatographic methods to one work day opposed to one work week with conventional HPLC.
A systematic approach towards chromatographic methods development that monitors selectivity change in a separation by manipulating pH, column chemistry and organic modifier was described. By utilizing UPLC Technology for methods development, a 6-fold improvement in throughput can be realized. This, in turn, reduces cost per sample and time of analysis considerably while maintaining or improving separation integrity. By developing rapid, high resolution analytical methods, products can be brought to market faster, therefore, improving the overall profitability of the assay.
720002338, April 2018