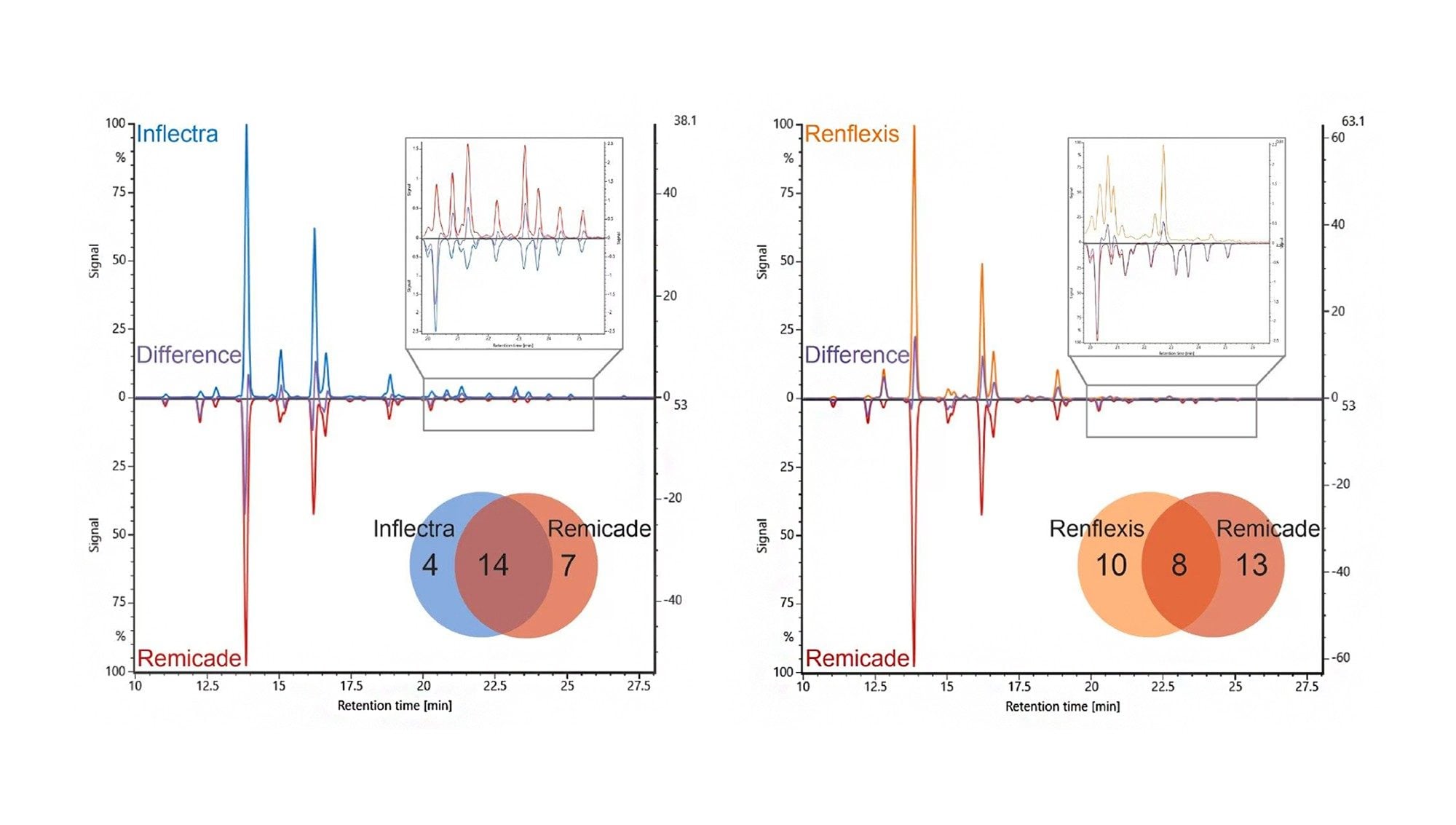
Global regulatory bodies require sufficient analytical and biological evidence of comparability between reference product and biosimilar candidates. Labs must characterize multiple batches of proteins to establish the biosimilarity of a therapeutic drug candidate to the original reference product molecule.
Waters integrated solutions provide a comprehensive platform for streamlined and robust analysis of the product attributes of biosimilars and their reference products, including:
Partner with Waters to support therapeutic safety and efficacy and improve patient health by confidently establishing comparability of biosimilar products.

Glycosylation significantly influences the properties of therapeutic proteins, including efficacy and immunogenicity. By understanding and controlling glycosylation patterns, biosimilar developers can demonstrate equivalent product quality and consistency during manufacturing.
Ensuring that biosimilars are equivalent to innovator drugs in terms of glycosylation is crucial for regulatory approval and patient safety. Waters provide market leading tools for analysis of glycopeptides and released N-glycans, providing an accurate picture of differences in major and minor glycoforms to assess acceptable differences for process control and comparability.
Glycosylation significantly influences the properties of therapeutic proteins, including efficacy and immunogenicity. By understanding and controlling glycosylation patterns, biosimilar developers can demonstrate equivalent product quality and consistency during manufacturing.
Ensuring that biosimilars are equivalent to innovator drugs in terms of glycosylation is crucial for regulatory approval and patient safety. Waters provide market leading tools for analysis of glycopeptides and released N-glycans, providing an accurate picture of differences in major and minor glycoforms to assess acceptable differences for process control and comparability.
Webinar: Deploying Waters Solutions to Enhance Biosimilar Development

Streamline biosimilar analysis workflows and unite LC-MS systems across organizations with waters_connect for biopharmaceuticals, a compliant-ready, networked informatics platform.
BioResolve RP mAb Polyphenyl Columns provide improved peak capacity and minimal carry-over for intact and subunit analysis of proteins and mAbs specifically QC-tested with a NIST-based, mAb subunit standard. BioResolve SCX mAb Columns and Buffers reliably separate charge variants for a wide range of mAbs using optical or LC-MS compatible mobile phases.
Minimize secondary ionic or hydrophobic interactions in size-based separations with ACQUITY and XBridge Premier Protein SEC 250 Å Columns with MaxPeak High Performance Surface (HPS) Technology for reliable protein aggregate, monomer, and fragment analyses using a “platform” type method with 30% increase in resolution.
Gain enhanced recovery and sensitivity for acidic peptides with Waters ACQUITY, XSelect, and XBridge Premier Peptide Columns that are QC batch-tested with Waters Cytochrome c Digest Standard for increased column-to-column performance consistency.
Achieve quick, clean, and complete mAb and biosimilar digestions in just 30 minutes with RapiZyme Trypsin, Mass Spectrometry (MS) Grade, a highly activie autolysis-resistant enzyme that reduces the likelihood of artificial modifications occurring during sample preparation prior to LC-MS analysis.
Achieve high efficiency, reproducible peptide maps in under 2.5 hours with PeptideWorks Tryptic Protein Digestion Kits for manual or automated workflows that are 4x faster than average home-brew methods and provide a 78% reduction in missed cleavages.
Obtain fast, high resolution glycan identification for your biosimilars with Waters complete line of glycan columns to analyze glycoproteins, glycopeptides and released N-glycans with exceptional reproducibility.
Achieve unprecedented fluorescent and mass spectrometric performance for glycan detection with GlycoWorks N-glycan Kits that improve the throughput of N-glycan sample preparation so you don’t have to compromise between speed and sensitivity.
Fast, efficient, and reproducible online pepsin digestion is critical in experiments that require more peptide coverage. With the Waters Enzymate BEH Pepsin Column, you can easily incorporate online protein digestion into existing HDX- MS workflows. The Enzymate BEH Pepsin Column efficiently digests intact proteins into peptides in an online HDX system, typically in about 30 seconds.
Streamline sample preparation for LC-MS analyses with Waters Laboratory Automation and Equipment that deliver verified workflows to help minimize variability, improve traceability, and simplify method transfer.
Optimize your laboratory's productivity and success with Waters Global Services to maintain peak system performance, minimize downtime, address application challenges, and support stringent compliance requirements.
Maximize resources and minimize risk with payment options from Waters Capital, including upgrading aging equipment, getting customized support, and bundling services into one monthly payment.