LC-MS/MS Analysis of Immunosuppressant Drugs in Whole Blood using the Xevo™ TQ Absolute with the Capitainer® B Device for Clinical Research
For research use only. Not for use in diagnostic procedures.
Abstract
Here we describe a clinical research method using liquid-liquid extraction from a dried blood spot, itself collected from a small volume of pipetted human whole blood.
Benefits
- Analytical sensitivity from low volume samples using the Xevo TQ Absolute
- Fast analytical run time, with simultaneous analysis of four analytes, afforded by the analytical selectivity of the chromatography and mass detection
- Small sample volume with a Capitainer B device provided good precision and accuracy
Introduction
Deprez et al have previously demonstrated an accurate, reproducible LC-MS/MS method for the analysis of cyclosporine, everolimus, sirolimus, and tacrolimus from a small volume of whole blood, by pipetting onto the inlet of a Capitainer B Device in order to generate a dried blood spot of known volume.1
Here we describe a clinical research method using liquid-liquid extraction from a dried blood spot, itself collected from a small volume of pipetted human whole blood. Chromatographic elution using a Waters™ ACQUITY™ UPLC™ HSS C18 SB Column on a Waters ACQUITY UPLC I-Class PLUS was completed within 1.5 minutes. Simultaneous analysis of all four analytes, followed by detection on a Xevo TQ Absolute Mass Spectrometer (Figure 1) was achieved with a time of 2.2 minutes from injection-to-injection.

Experimental
Sample Preparation
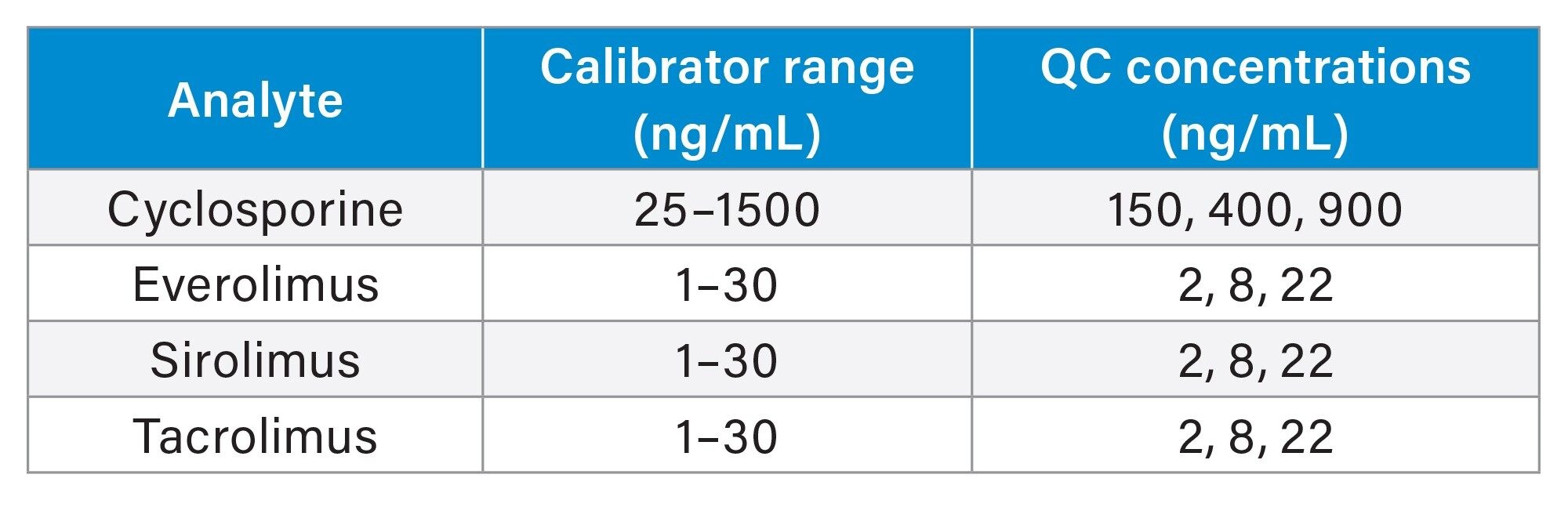
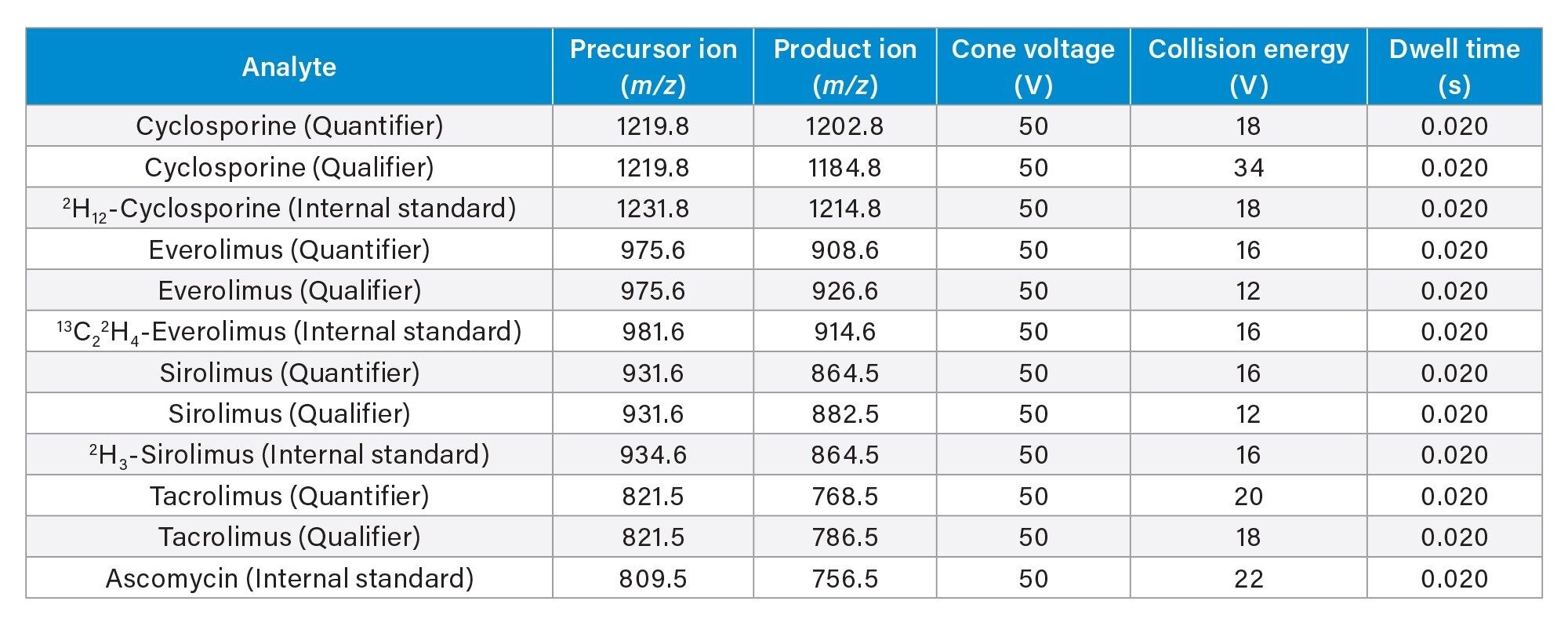
Whole blood MassTrak Immunosuppressant Calibrators and Quality Control Sets containing cyclosporine, everolimus, sirolimus and tacrolimus were used. Respectively, 2H12-cyclosporine, 13C22H4-everolimus, 2H3-sirolimus, and ascomycin were used as internal standards.
The calibrator and QC concentrations (at low, medium, and high concentrations) are detailed in Table 1.
Sample Extraction
Pipette 30 µL sample onto the inlet of the device, and check that a viable, 10 µL, dried blood spot is formed, then allow to dry overnight. Transfer the dried blood spot to a microcentrifuge tube, add 200 µL working internal standard, then mix on a multi-tube vortex mixer (2500 r.p.m. for 30 minutes), sonicate (20 minutes), and a final vortex mix (2500 r.p.m. for 10 minutes). Add 10 µL of 0.05M hydrochloric acid and 1 mL tert-methyl butyl ether. Mix on a multi-tube vortex mixer (2500 r.p.m. for three minutes) and centrifuge at 25155 g for two minutes. Transfer 850 µL of the upper layer to a total recovery vial (p/n: 186005669CV) and evaporate to dryness under a stream of nitrogen at 40 °C. Reconstitute in 200 µL of mobile phase A:mobile phase B 50:50 (v:v).
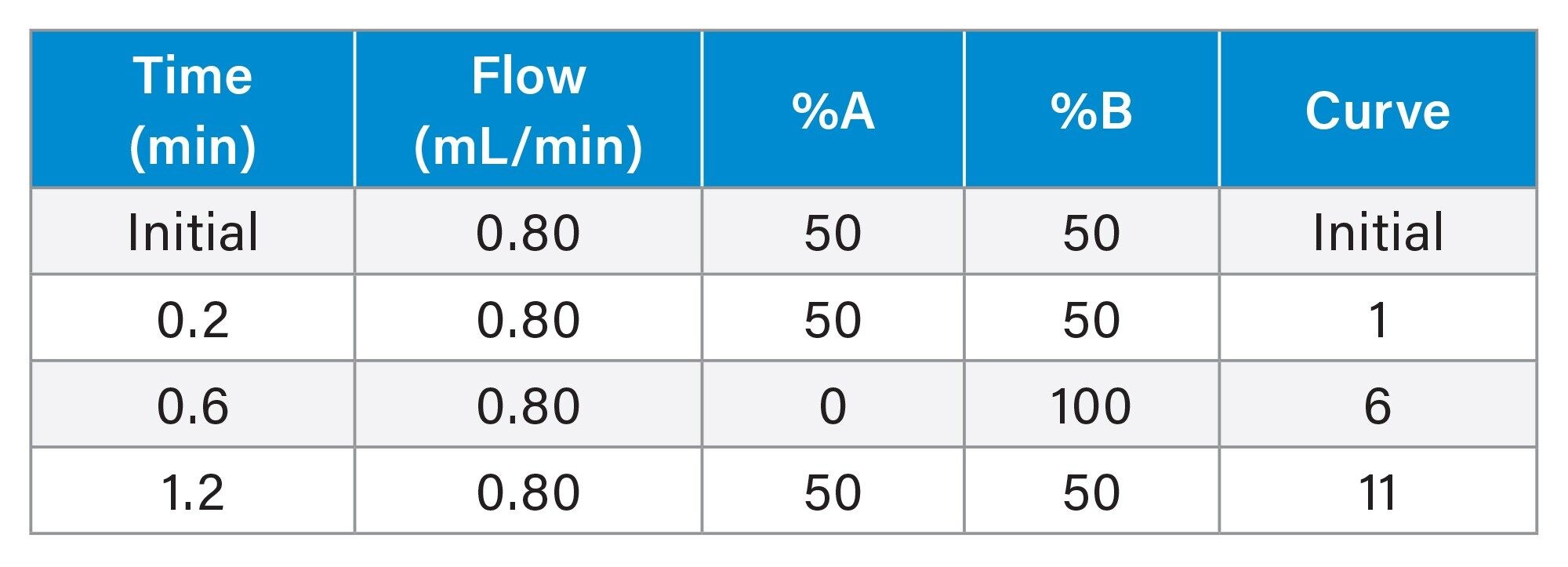
UPLC Conditions
|
System: |
ACQUITY UPLC I-Class PLUS with FL |
|
Needle: |
20 µL |
|
Loop: |
50 µL |
|
Column: |
ACQUITY UPLC HSS C18 SB Column; 1.8 µm, 2.1 x 30 mm (p/n: 186004117) |
|
Column temperature: |
55 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
20 µL |
|
Injection mode: |
Partial Loop, with Load Ahead disabled |
|
Mobile phase A: |
Water + 0.05 mM ammonium fluoride |
|
Mobile phase B: |
Methanol + 0.05 mM ammonium fluoride |
|
Weak wash: |
Water:methanol 95:5 (v:v), 600 µL |
|
Strong wash: |
Water:methanol:acetonitrile:IPA 25:25:25:25 (v:v:v:v), 200 µL |
|
Seal wash: |
Water:methanol 80:20 (v:v) |
|
Run time: |
1.5 minutes (2.2 minutes) |
MS Conditions
|
System: |
Xevo TQ Absolute |
|
Resolution: |
MS1 (0.7 FWHM) MS2 (0.7 FWHM) |
|
Acquisition mode: |
Multiple Reaction Monitoring (MRM) (see Table 3 for details) |
|
Polarity: |
ESI positive ionization (ESI+) |
|
Capillary: |
1.0 kV |
|
Source temperature: |
120 °C |
|
Desolvation temperature: |
400 °C |
|
Inter-scan delay: |
0.003 seconds |
|
Inter-channel delay: |
0.02 seconds |
Data Management
MassLynxTM v4.2 with TargetLynx™ Application Manager.
Results and Discussion
Five analytical runs were performed using this method.
Figure 2 shows example chromatograms of calibrator 1 (25 ng/mL cyclosporine and 1 ng/mL everolimus, sirolimus, and tacrolimus).
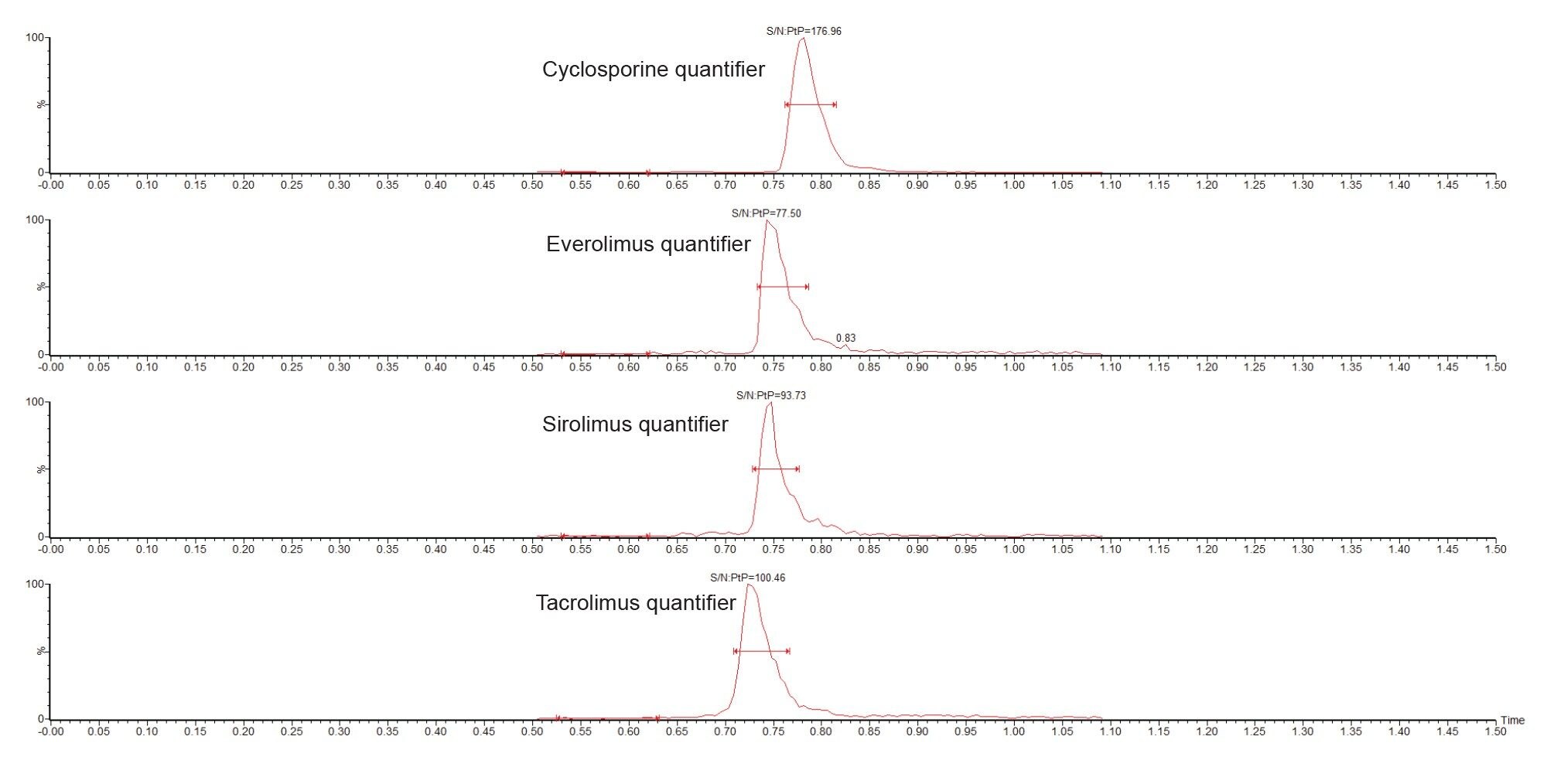
Analytical sensitivity of the lowest calibrators was demonstrated with S/N (PtP) > 10:1 across the five analytical runs (Table 4).
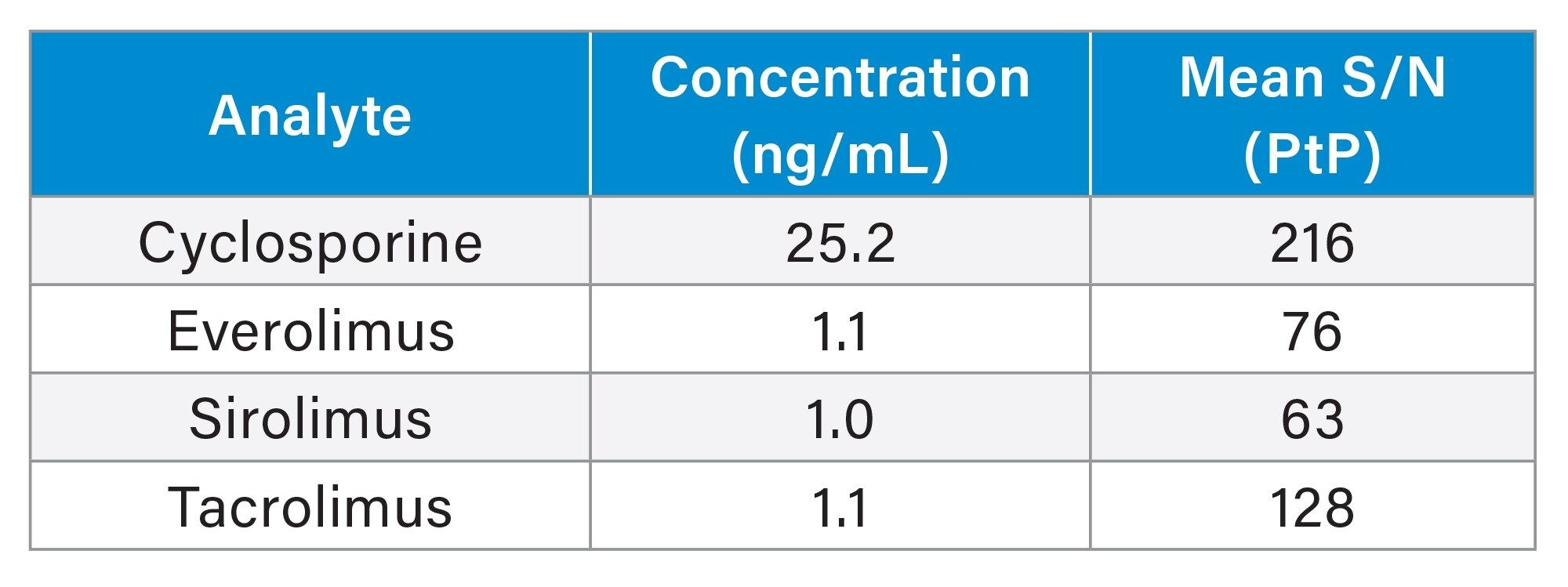
No system carryover was observed in each run following analysis of a calibrator 6 sample with a blank sample for cyclosporine, everolimus, sirolimus, and tacrolimus.
The calibration curves over five analytical runs were linear with r2 ≥0.99 for each of the immunosuppressants over their calibration ranges (presented in Table 1).
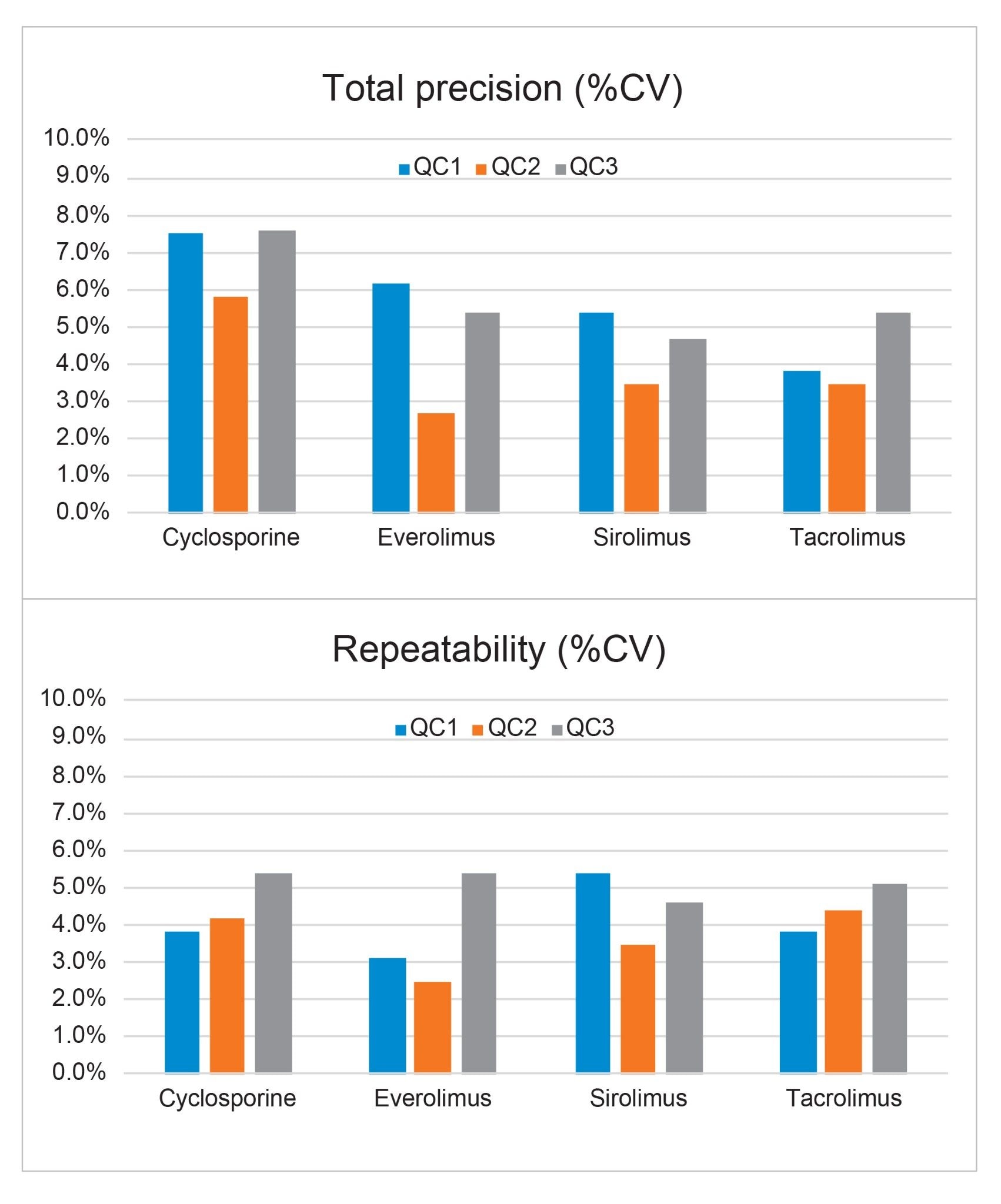
Total precision was determined by extracting and quantifying five replicates of three concentrations of whole blood pools over five separate days (n=25). The exception was for cyclosporine, for which there were four separate days (n=20). Repeatability was assessed by analysing five replicates at each QC level. Figure 3 presents results of these experiments, where total precision and repeatability at the three concentrations assessed was ≤7.6% RSD.
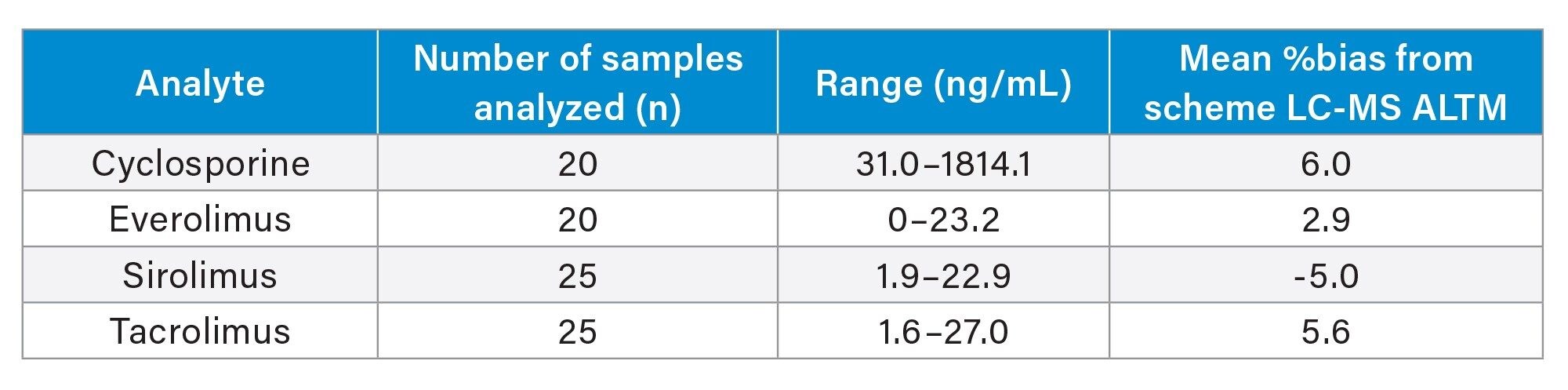
LGC (Bury, UK) whole blood External Quality Assurance samples were sourced and analysed to assess method accuracy. A summary is presented in Table 5.
Conclusion
Simultaneous analysis of the immunosuppressant drugs cyclosporine, everolimus, sirolimus and tacrolimus can be achieved in a single analysis of a little over two minutes (injection-to-injection), using a very small whole blood volume together with a Capitainer B device to generate a dried blood spot.
The performance characteristics of the method indicate good analytical sensitivity, total precision, and repeatability (≤7.6% RSD) across all analytes and concentrations tested.
Finally, good agreement was obtained when analysing External Quality Assurance samples, providing confidence in the accuracy of the method.
Acknowledgements
Capitainer are thanked for the provision of Capitainer B devices for this study.
1Deprez S, Van Uytfanghe K and Stove CP. Liquid chromatography-tandem mass spectrometry for therapeutic drug monitoring of immunosuppressants and creatinine from a single dried blood spot using the Capitainer qDBS device. Analytica Chimica Acta. 1242 (2023) 340797.
720007893, March 2023