A Look at Column Reproducibility: Analysis of Endogenous Steroid Hormones in Serum Using Three Historical Batches of 1.8 µm HSS T3 Stationary Phase
Abstract
Batch to batch testing of stationary phases for a given assay is a common method robustness experiment.1-3 Having reproducible columns is critical for the success of these tests, as well as ensuring lifelong performance for the assay. To demonstrate the reproducibility of Waters column chemistries, the analysis of endogenous steroid hormones was performed on three batches of ACQUITY UPLC HSS T3 manufactured over a five-year span. Low %RSD values for retention time, peak area, peak width, and peak symmetry demonstrate that the HSS T3 stationary phase is highly reproducible, and little to no chromatographic differences were seen between the oldest and newest batches.
Benefits
- Comparable batch to batch performance for three historical batches of HSS T3 material
- Improved signal and detection using Solid Phase Extraction over Protein Precipitation with ACQUITY UPLC HSS T3 columns
Introduction
Having high quality products, particularly liquid chromatography (LC) stationary phases and columns, ensures that the data generated for an assay is accurate and reproducible over time. The quality of LC stationary phases can be measured by batch-to-batch reproducibility testing for a given assay to ensure that each manufactured lot of material performs similarly. Most batch-to-batch tests are performed using three batches of material made within a certain time period, such as those within a method validation kit. While these materials can work well, their use may only provide a snapshot of the overall quality of the product. To get the full picture, historical batches of material should be tested, as this provides a better scope of overall product quality.
The ACQUITY HSS T3 stationary phase is a commonly used C18 bonded phase using fully porous silica particles. This phase is designed to retain polar analytes better than a traditional C18 without compromising the separation of hydrophobic compounds. This phase has been used in many applications and publications for separations ranging from small molecule pharmaceuticals in dosage form to bioanalytical applications.4-6 To demonstrate the quality of the HSS T3 material, three columns were packed with different batches of material. These materials were made five years apart ranging from 2016 to 2021. All three columns were then used to separate endogenous steroid hormones in human serum to assess the reproducibility of the data across all columns.
Experimental
Sample Description
Human serum was prepared by solid phase extraction (SPE) and protein precipitation (PPT). PPT using acetonitrile (3:1 acetonitrile:serum) was performed. Samples were vortexed, centrifuged, and the supernatant removed for analysis. SPE was performed using Oasis HLB 1 cc 30 mg cartridges (p/n: WAT094225). Sorbent conditioned with methanol and equilibrated with water as outlined in the literature.7 Generic SPE conditions were used, and elution performed using 100% acetonitrile. The eluate was dried under nitrogen at 60 °C and reconstituted in 100 µL Milli-Q water for analysis.
LC Conditions
|
LC systems: |
ACQUITY UPLC I-Class PLUS with Binary Solvent Manager (BSM), Sample Manager Flow Through Needle (SM-FTN), and PDA Detector |
|
Detection: |
MRMs of Serum Hormones outlined in Table 1 |
|
Column(s): |
ACQUITY UPLC HSS T3 1.8 µm, 2.1 x 50 mm (p/n: 186009467) Three (3) batches of material: 189 – Manufactured Oct-2016 233 – Manufactured Nov-2019 270 – Manufactured Nov-2021 |
|
Column temp.: |
30 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
5.0 µL |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
0.1% Formic acid in Milli-Q Water |
|
Mobile phase B: |
0.1% Formic acid in acetonitrile |
|
Gradient profile: |
5% to 95% B in 5.0 minutes, hold at 95% B for 0.5 minutes. |
MS Conditions
|
MS system: |
Xevo TQ-S micro |
|
Ionization mode: |
ESI+ |
|
Acquisition range: |
MRMs outlined in Table 1 |
|
Capillary voltage: |
3.0 kV |
|
Cone voltage: |
Analyte dependent (Table 1) |
|
Desolvation temp.: |
500 °C |
|
Desolvation flow: |
500 L/hr |
|
Cone gas flow: |
10 L/hr |
Data Management
|
Chromatography software: |
MassLynx v4.1 |
Results and Discussion
Prior to column testing two different sample preparation techniques were assessed to optimize the assay. PPT and SPE were performed on a sample of human serum. The SPE protocol outlined in the experimental section using Oasis HLB 1 cc 30 mg cartridges was not optimized for this experiment. Generic SPE conditions were used to demonstrate effectiveness of SPE compared to PPT. Further improvements in signal could be achieved by optimization of SPE protocol. Both sample preparation techniques were analyzed using a single batch of ACQUITY UPLC HSS T3 1.8 µm, 2.1 x 50 mm to evaluate mass spectrometry (MS) signal intensity of expected analytes. Figure 1 shows the comparison of SPE vs PPT results for the steroid hormones detected in serum.
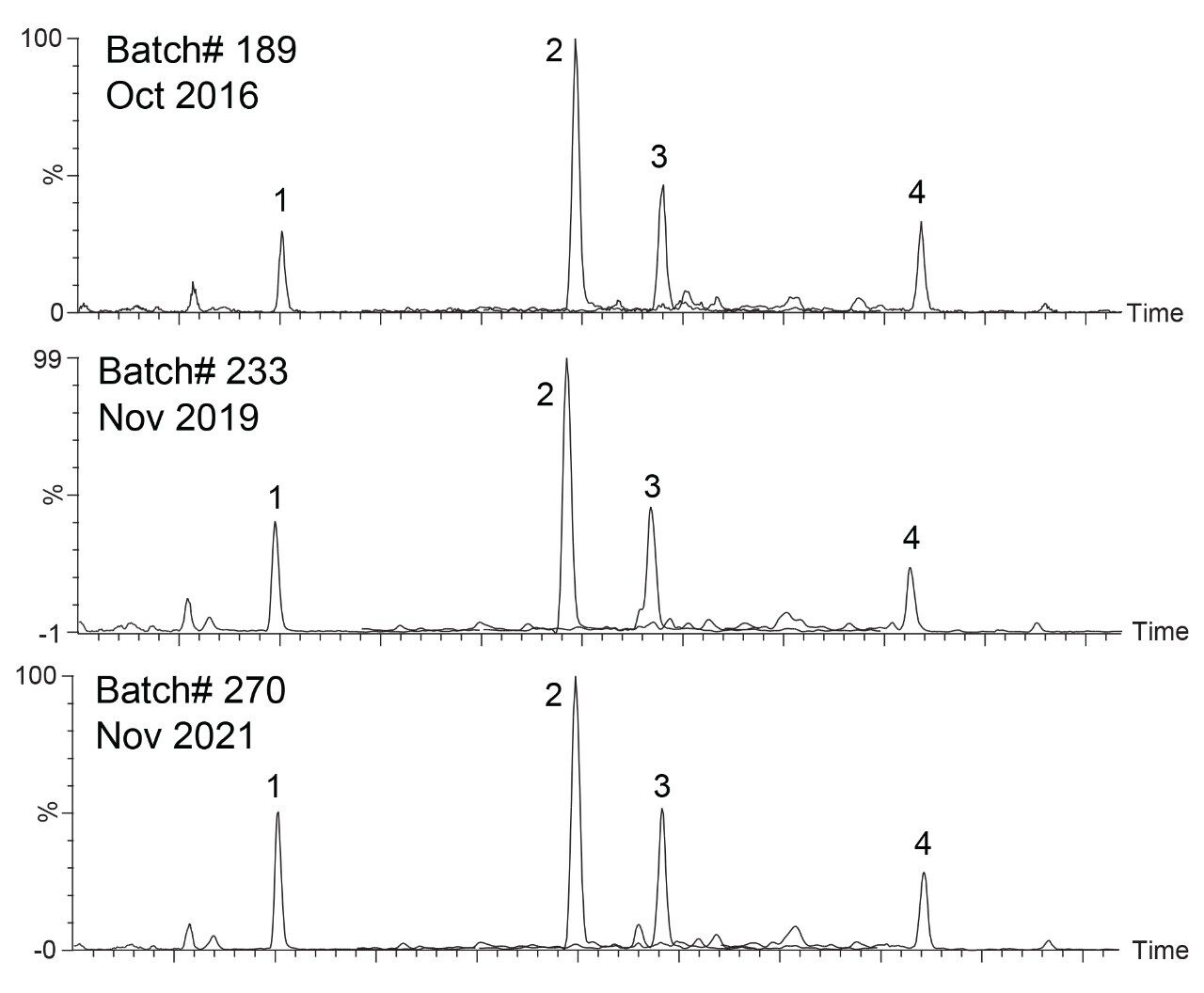
A panel of potential steroids in serum were examined.8 Four steroid hormones were detected with signal >1e5 after SPE under these conditions. The remaining steroids were not detected. Peak identification was performed using a standard mixture of the steroids and the test conditions indicated. The results after PPT show only two steroid hormones, testosterone, and cortisol, with considerably lower signals compared to SPE. Androstenedione and progesterone were not detected after PPT. SPE produced a 5x increase in signal for cortisol and about a 30x increase in signal for testosterone. This provides not only a more easily detectable peak, but also reduces the LOD and LOQ of the assay and can give a wider dynamic range for SPE recovery studies. SPE also creates a cleaner sample by removing phospholipids and other problematic compounds.9,10 Cleaner samples reduce the rate of instrument and column fouling while also reducing matrix effects such as ion suppression or enhancement. Moving forward to column comparisons SPE will be used for sample preparation.
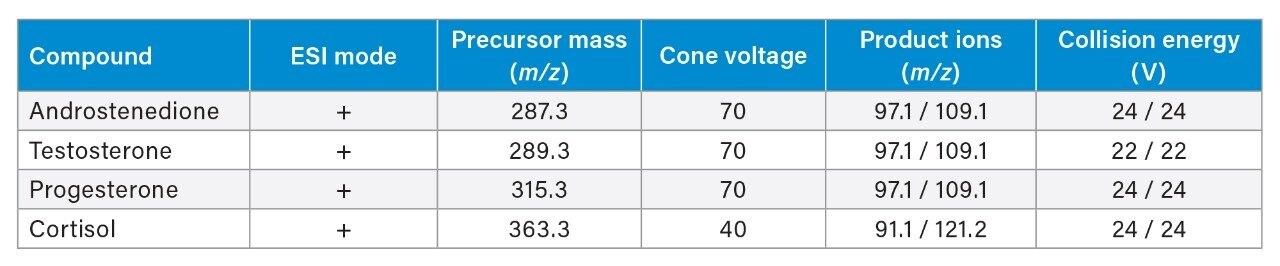
Fresh samples were prepared by SPE to evaluate three historical batches of ACQUITY UPLC HSS T3 1.8 µm material. The batches were created over a five-year span, with the oldest batch being manufactured and tested in October 2016. The remaining batches were manufactured in November 2019 and November 2021. Figure 2 shows the chromatograms of the four endogenous steroids on the three columns. Replicate injections (n=3) of serum were performed on each column, with two blanks before and after to ensure there was no system carryover.
1) Cortisol, 2) Testosterone, 3) Androstenedione, and 4) Progesterone.
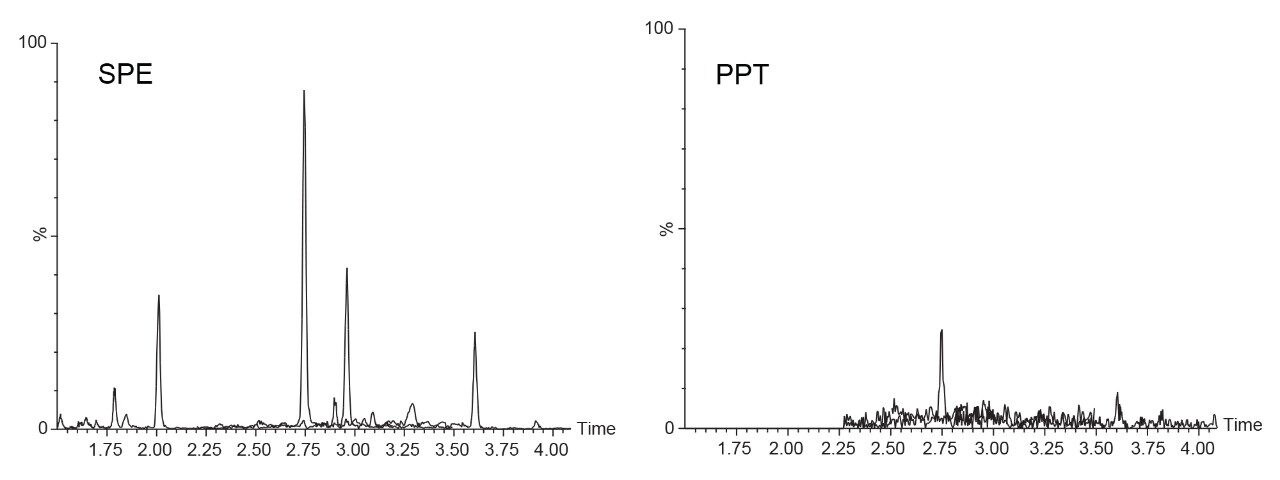
All three columns achieve comparable results for the analysis of endogenous steroids. Peak retention times, peak areas, peak widths, and peak symmetry were monitored and assessed for all three columns, as well as pooled data samples, Table 2. %RSDs for retention time were <1% for all probes on each column (n=3) as well as the pooled data set (n=9). Peak area reproducibility was good with a %RSD <5% for each column and pooled data samples. Similarly, low %RSDs were obtained for peak width (half height) and peak symmetry.
Good batch-to-batch reproducibility for this assay is achieved even with materials that were manufactured over a five-year span. Using materials with this level of reproducibility ensures that an assay will perform the same over its lifetime which can be several years. Waters columns provide this level of reproducibility through stringent quality control and manufacturing processes, to ensure the data generated on those columns is of the highest possible quality.
Conclusion
To demonstrate the quality of Waters particle and column manufacturing processes, three batches of ACQUITY HSS T3 stationary phase, manufactured over a five-year span, were used to separate endogenous steroid hormones in human serum. SPE was used for all sample preparation for reproducibility testing.
The three batches showed reproducible chromatographic performance. %RSD values for retention time were <1% for each column as well as the pooled data set (n=9). Additionally, %RSDs for peak area, peak width, and peak symmetry were <5% for each detected analyte. These results indicate that even though the materials were made five years apart, the same quality of data can be generated. This level of assurance, that similar data can be achieved over years, is critical when developing new methods. Knowing that the selected stationary phase will be the same lot-to-lot and year-to-year allows for a longer assay lifetime without the need for additional rework.
Acknowledgements
Jonathan Danaceau, Chelsea Plummer, Jonathan Turner, and Kim Haynes for reviewing the application note and providing valuable feedback.
References
- Heyden YV, Nijhuis A, Smeyers-Verbeke J, Vandeginste BGM, Massart DL. Guidance for Robustness/Ruggedness Tests in Method Validation. Journal of Pharmaceutical and Biomedical Analysis. 2001. 723–753.
- Vervoort RJ, Ruyter E, Debets A, Claessens HA, Cramers C, de Jong GJ. Influence of Batch-To-Batch Reproducibility of Luna c18 (2) Packing Material, Nature of Column Wall Material, and Column Diameter on the Liquid Chromatographic Analysis of Basic Analytes. Journal of Separation Science. 2001. 167–172.
- Summers M, Fountain K. Validation of a Method for the Separation of Ziprasidone and Its Degradants using Empower 2 with Method Validation Manager (MVM). Waters Application Note, 720004077, 2011. Accessed 10-Jan-2022.
- New LS, Chan ECY. Evaluation of BEH C18, BEH HILIC and HSS T3 (C18) Column Chemistries for the UPLC-MS-MS Analysis of Glutathione, Glutathione Disulfide, and Ophthalmic Acid in Mouse Liver and Human Plasma. Journal of Chromatographic Science. 2008. 209–214.
- Mohamed, M. Stability Indicating New RP-UPLC Method for Simultaneous Determination of a Quaternary Mixture of Paracetamol, Pseudoephedrine, Chlorpheniramine, and Sodium Benzoate in (Cold-Flu) Syrup Dosage Form. Journal of AOAC International. 2022.
- Sottani C, Grignani E, Cottica D, et. al. Development and Validation of a Bioanalytical UHPLC-MS/MS Method Applied to Murine Liver Tissue for the Determination of Indocyanine Green Loaded in H-Ferritin Nanoparticles. Frontiers in Chemistry 2022.
- Elmongy H, Masquelier M, Ericsson M. Development and Validation of a Uhplc-Hrms Method for the Simultaneous Determination of the Endogenous Anabolic Androgenic Steroids in Human Serum. Journal of Chromatography A. 2019. 1613.
- Foley D, Carlton LJ. Confidence in Your Calibrators: Confidence in Your Calibrators: MassTrak Endocrine Steroid Calibrators and Quality Control Sets for the LC-MS/MS Analysis of Steroid Hormones. Waters Application Note, 720007401, 2021. Accessed 10-Jan-2021.
- Zhang X, Danaceau JP, Chambers EC. Quantitative Analysis of THC and its Metabolites in Plasma Using Oasis PRiME HLB for Toxicology and Forensic Laboratories. Waters Application Note, 720005820, 2019. Accessed 13-Jan-2022.
- Zhang X, Danaceau JP, Chambers EC. Simple, Fast, and Clean Extraction of Synthetic Cannabinoids from Whole Blood Using Oasis PRiME HLB. Waters Application Note, 720005417, 2015. Accessed 14-Jan-2022.
720007529, February 2022