Achieving insights into the structure of proteins and protein macromolecular complexes is critical in understanding the relationship with their biological function.
Using either HDX-MS or Native MS analysis, the SELECT SERIES Cyclic IMS can help your lab learn about the physical size and shape of proteins, and is ideal for modeling protein assembly structures that are difficult and time-consuming to study with conventional structural biology approaches.
For studying protein unfolding and subunit configurations, the Cyclic IMS can be configured with an array of ion activation techniques, including electron capture dissociation (ECD), surface induced dissociation (SID), and collision-induced dissociation (CID). All of these techniques combine with scalable ion mobility and IMSn to deliver unique insights in structure.


Significantly minimize or remove the introduction of extraneous salt ions into the mass spectrometer with minimal sample handling using MassPREP On-Line Desalting Cartridges.
Easily incorporate online protein digestion into existing HDX-MS workflows with Enzymate BEH Pepsin Columns, which can typically digest intact proteins into peptides in an online HDX system in about 30 seconds.
Get unmatched batch-to-batch reproducibility with the wide range of Waters Size-Exclusion Chromatography (SEC) Columns and standards for protein and peptide characterization.
Achieve the efficiency and selectivity required for complex separations like the discovery of low abundance species, peptide mapping, protein identification, and biomarker discovery with Nano and MicroFlow LC-MS Columns.
Optimize your laboratory’s productivity while addressing your budget realities with Waters Global Services. Maintain peak system performance, minimize down time, address scientific application challenges, and support stringent compliance requirements.
Maximize your lab resources and minimize risk with payment options from Waters Capital, which includes innovative solutions to upgrade aging instruments, customized support, and flexible options to bundle your complete laboratory solution in one easy monthly payment – everything you need to advance your science.
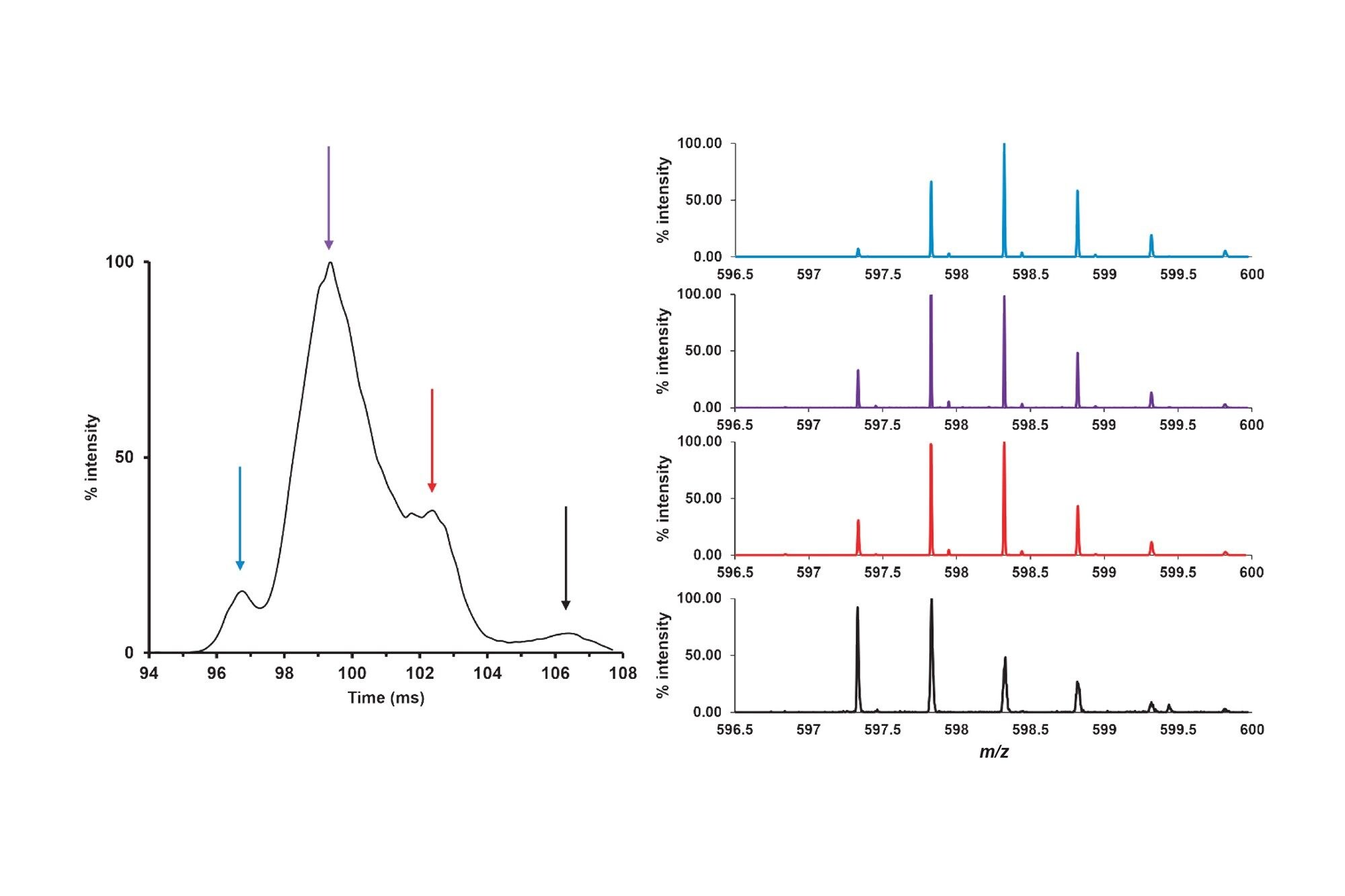

Resolving deamidations for confident quantification. A) The deamidated forms of peptide HC:T23 elute in the tail of the native peptide peak.
Without the ultra-high resolution of the SELECT SERIES MRT as shown in (B) identification and quantification of the deamidations.
Structural biology is a field of science that focuses on understanding the shapes and arrangements of biological molecules such as proteins, DNA, RNA, and large molecular complexes. These molecules are essential for life, and their structure is closely tied to their function. Even small changes in their shape, caused by misfolding or genetic mutations, can lead to diseases. By studying these molecular structures, scientists gain valuable knowledge about how biological systems work at the most basic level.
Researchers use advanced techniques to study these molecules in detail. X-ray crystallography provides highly detailed images of crystallized molecules, while Nuclear Magnetic Resonance (NMR) spectroscopy allows scientists to examine molecules in liquid form and study how they move. Alongside these, mass spectrometry and liquid chromatography–mass spectrometry (LC-MS) are widely used to study how molecules interact and change shape.
The knowledge gained from structural biology is extremely valuable in medicine and biotechnology. It helps researchers design new drugs that precisely target the structure of disease-related proteins. This is particularly important in diseases linked to protein misfolding. Beyond healthcare, structural biology also supports biotechnology and synthetic biology, where scientists develop or redesign proteins for industrial, environmental, or therapeutic uses.