This is an Application Brief and does not contain a detailed Experimental section.
This Application brief demonstrates the performance capabilities and reproducibility of microscale 2D-RP/RP peptide chromatography with an ACQUITY UPLC M-Class System and Column.
2D-RP/RP with the ACQUITY M-Class System and Columns reliably generates the high peak capacity separations needed to study complex peptide samples.
Microscale LC-MS methods have been used extensively in the field of proteomics. Recently, these techniques have become increasingly attractive as orthogonal methods alongside immunoassays for the analysis of host cell protein impurities in biotherapeutics products. Narrow (300 μm ID) columns can be employed in these and other such applications as a means to derive an abundance of information from a relatively minimal amount of sample. Obtaining high peak capacity peptide separations is desirable in such work, because better separation efficiency means traceanalytes can be more easily resolved.
High peak capacity peptide separations can be obtained through multidimensional chromatography wherein the combination of orthogonal separations results in greater resolving power. Two-dimensional reversedphase (2D-RP/RP) chromatography is a unique example. The utility of 2D-RP/RP involving high pH fractionation with a highly stable, organo-silica hybrid stationary phase (BEH Technology) followed by gradient separation on a sub-2-μm particle analytical column has been reported previously.
Here, we demonstrate that 2D-RP/RP chromatography operated at ≥10K psi with an ACQUITY UPLC M-Class and an ACQUITY UPLC M-Class 300 μm analytical column can be a robust solution for obtaining high peak capacity. It will be shown that such a system affords high resolving power and outstanding chromatographic reproducibility and performance with extended use.
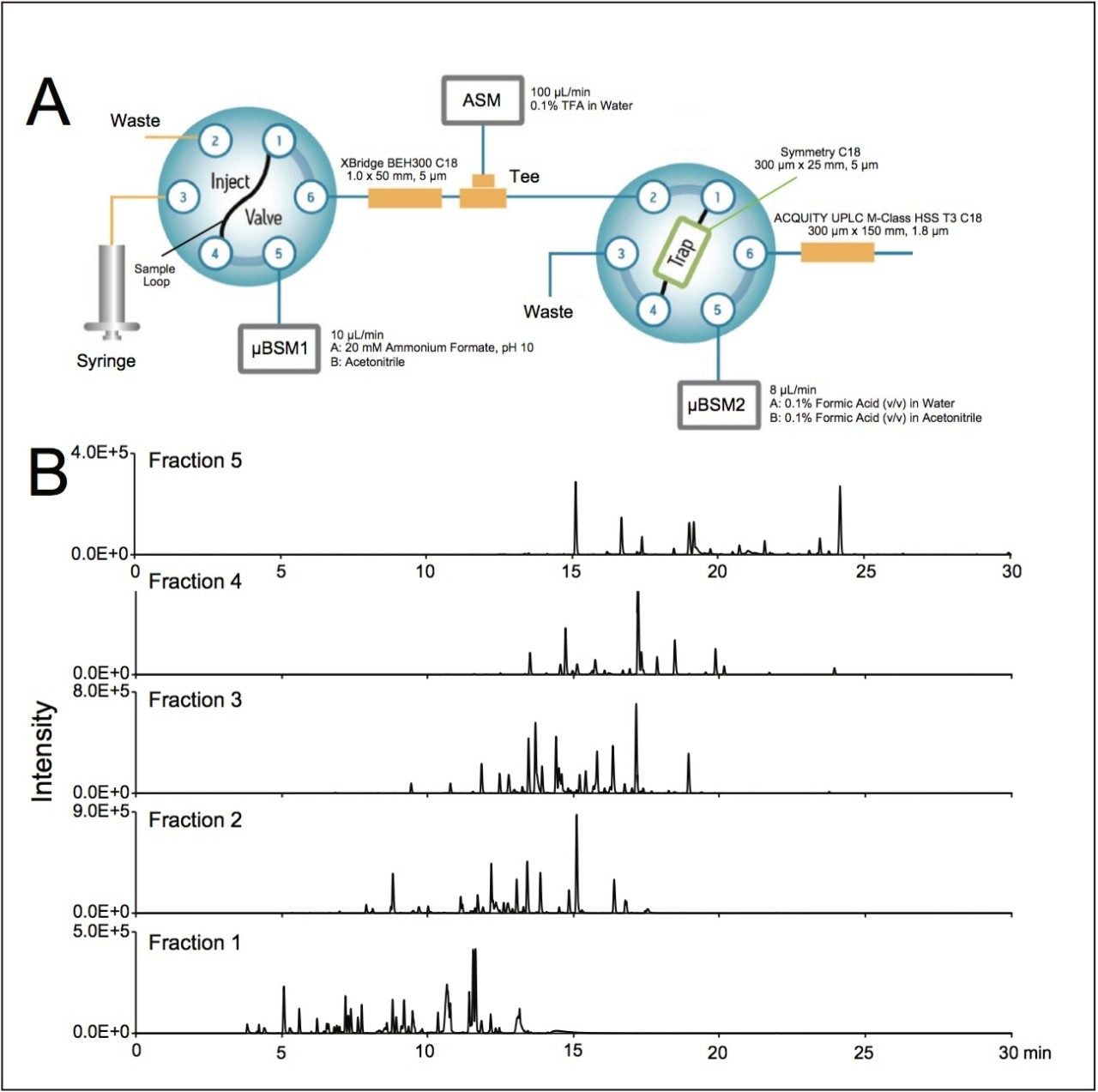
A tryptic digest mixture derived from 4 different proteins (MassPREP Digestion Standard Mix 1,) was studied by 2D-RP/RP using the configuration outlined in Figure 1A. Peptides from 400 fmoles of sample were separated by a linear gradient at a flow rate of 8 μL/min (10,000 psi) using an ACQUITY UPLC M-Class and detected by ESI-MS with a micro-probe outfitted SYNAPT G2-S at a resolution of ca. 20,000. Figure 1B presents base peak intensity chromatograms typical of this analytical strategy, wherein a 5-step fractionation was combined with bidirectional flow trapping and a 30 min 2nd dimension gradient with an ACQUITY UPLC M-Class HSS T3, 300 μm x 150 mm Column.
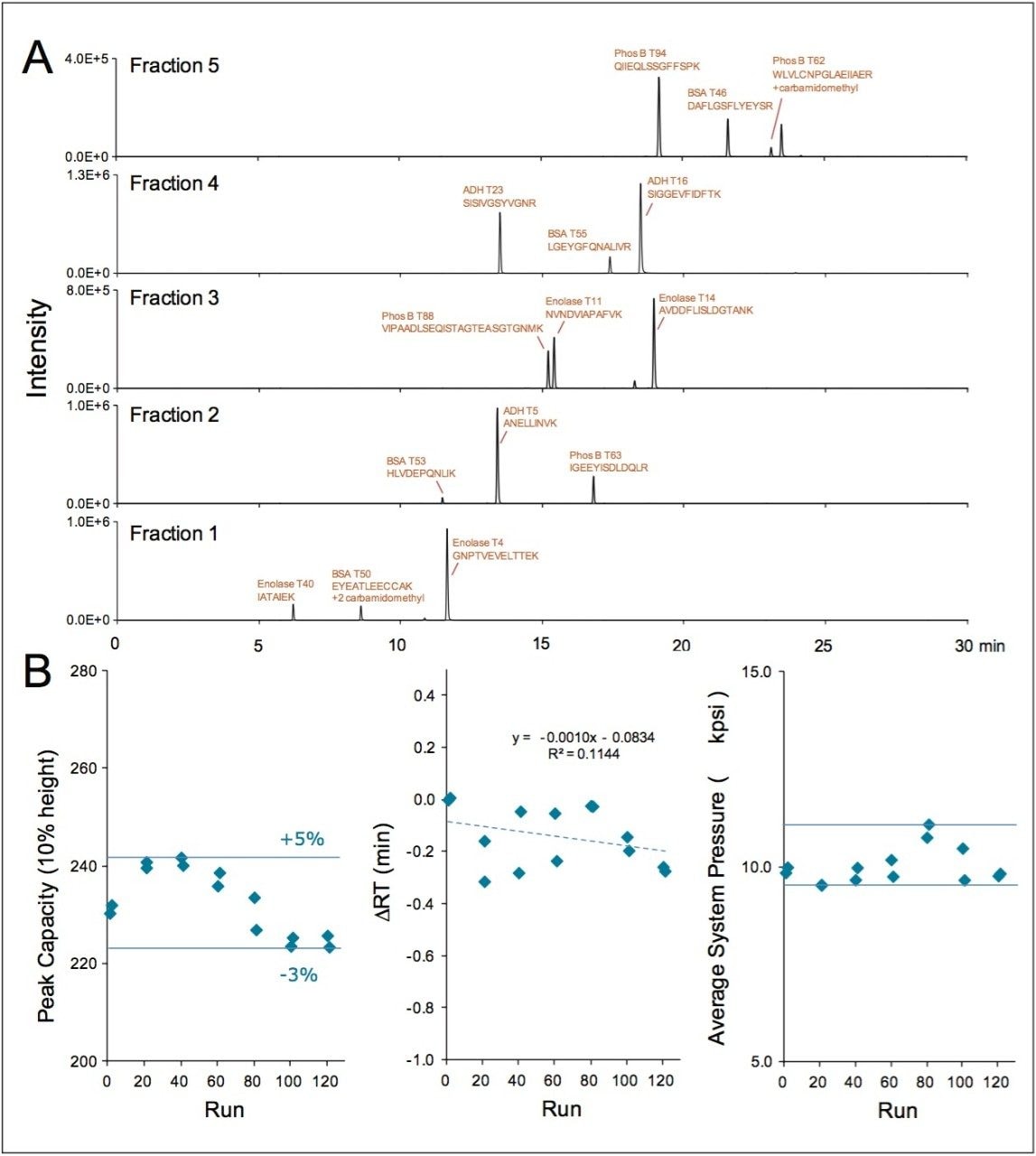
Based on peak widths observed in the extracted ion chromatograms of 15 different peptides in this mixture (Figure 2A), the average peak capacity (10% peak height) for the 2nd dimension separations was estimated to be 277. As these analyses were completed with 5 step fractionation, the multiplicative resolving power of the two dimensions indicates that the apparatus is capable of producing a theoretical peak capacity of 1385. This demonstrates that remarkably high peak capacity peptide separations can be achieved by means of 2D-RP/RP with an ACQUITY UPLC M-Class System. The reproducibility of this chromatography proved to be equally impressive. In studying the performance of three different column sets, it was found that from 6 replicate analyses the retention times of the 15 monitored peptides could be reported with a standard deviation of ≤0.11 min. Moreover, the largest difference in average retention time between the columns sets for a particular peptide was only 3.2%, and the peak capacities obtained with each column set were in agreement to within 20%. Lifetime testing of this 2D-RP/RP application also revealed the chromatography to be robust. Performance under extended use was investigated by effectively subjecting the columns to over 100 2D analyses. To expedite this life-time study, full gradient elution of the 2nd dimension column was only performed for 2 of every 20 cycles. This accelerated the aging of the high pH 1st dimension column as well as the trapping column, which is expected to represent the predominant mode of failure in the system. Data obtained in this manner for runs 1 to 121 are displayed in Figure 2B. Notably, the 2D-RP/RP method exhibited consistent peak capacities throughout the lifetime study (variation in peak capacity ≤5%), an average retention shift of a thousandth of a minute per run (0.1 min for 100 runs), and an average system pressure ranging from 9,500 psi to 11,100 psi, well below the 15,000 psi maximum operating pressure of the ACQUITY UPLC M-Class System.
Two dimensional RP comprised of high pH fractionation with a highly stable, organo-silica hybrid stationary phase (BEH Technology) followed by gradient separation on a sub-2-μm particle analytical column is a superb example of 2D chromatography. Its utility is expanded upon here, with an ACQUITY UPLC M-Class System and an ACQUITY UPLC M-Class 300 μm ID analytical column. Not only is the resolving power of such a system noteworthy, so too is its reproducibility and robustness, which has been evaluated through analysis of multiple column sets and accelerated lifetime testing. Two dimensional RP with the ACQUITY UPLC M-Class System and Columns holds significant promise as a means to reliably generate the high peak capacity separations needed to study complex peptide samples, such as those faced during host cell protein analysis.
720004934, January 2014