
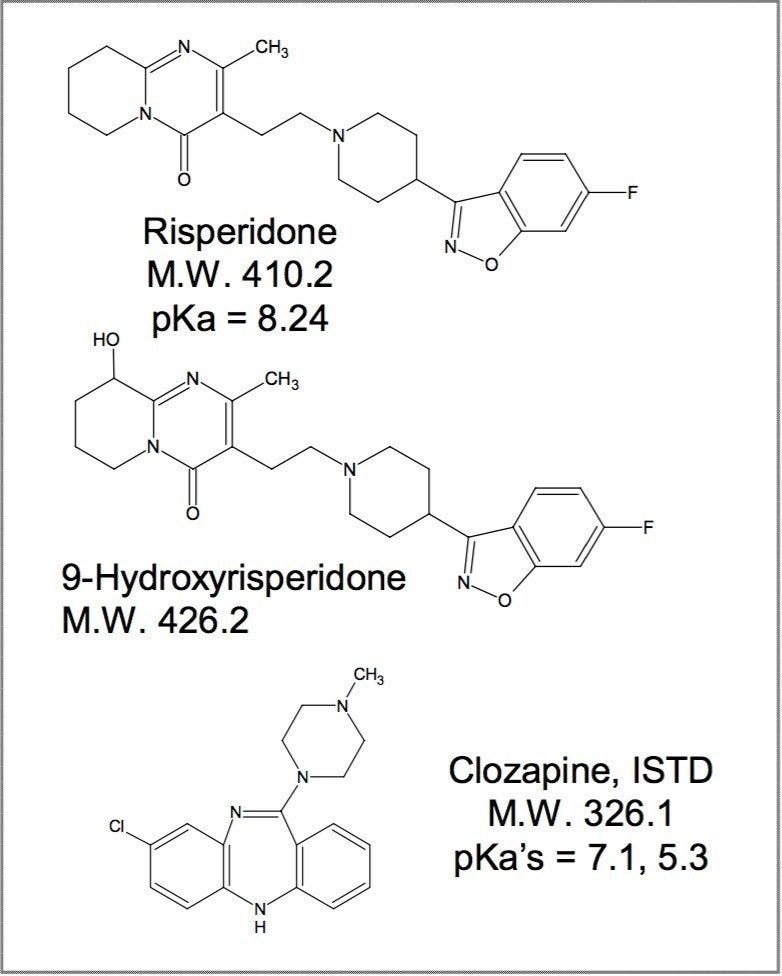
This application note details the full validation of a UPLC-MS/MS method for the determination of risperidone and its active metabolite 9-hydroxyrisperidone concentrations in human plasma, using clozapine as an internal standard.
Risperidone is from a class of compounds called atypical antipsychotics. Its main use is in the treatment of schizophrenia by blocking serotonin 5-HT2 and dopamine D2 receptors. Risperidone is rapidly absorbed by the body after administration and is metabolized to 9-hydroxyrisperidone. The monitoring authorities require the safety and efficacy of drugs and their active metabolites to be assessed. This application note details the full validation of a UPLC-MS/MS method for the determination of risperidone and its active metabolite 9-hydroxyrisperidone concentrations in human plasma, using clozapine as an internal standard (Figure 1). The full validation was carried out in accordance with FDA Guidance for Industry for Bioanalytical Method Validation and included the assessment of:
|
LC system: |
ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC BEH C18, 2.1 x 50 mm, 1.7 μm |
|
Mobile phase A: |
2 mM CH3COO–NH4+ in H2O, pH 9.0 |
|
Mobile phase B: |
Methanol |
|
Flow rate: |
0.6 mL/min |
|
Injection volume: |
5 μL |
|
Sample diluent: |
50:50 v/v methanol:water |
|
Column temp: |
50 °C |
|
Time(min) |
%A |
%B |
Curve |
|---|---|---|---|
|
0 |
50 |
50 |
- |
|
0.25 |
50 |
50 |
6 |
|
0.75 |
0 |
100 |
6 |
|
1.25 |
50 |
50 |
11 |
|
MS system: |
Quattro Premier XE Tandem Quadrupole Mass Spectrometer |
|
Ionization mode: |
Positive ion electrospray (ESI+) |
|
Capillary voltage: |
3.00 V |
|
Desolvation temp: |
380 °C |
|
Desolvation gas flow: |
800 L/hr |
|
Cone gas flow: |
50 L/hr |
|
Collision cell pressure: |
3.50 e-3 |
|
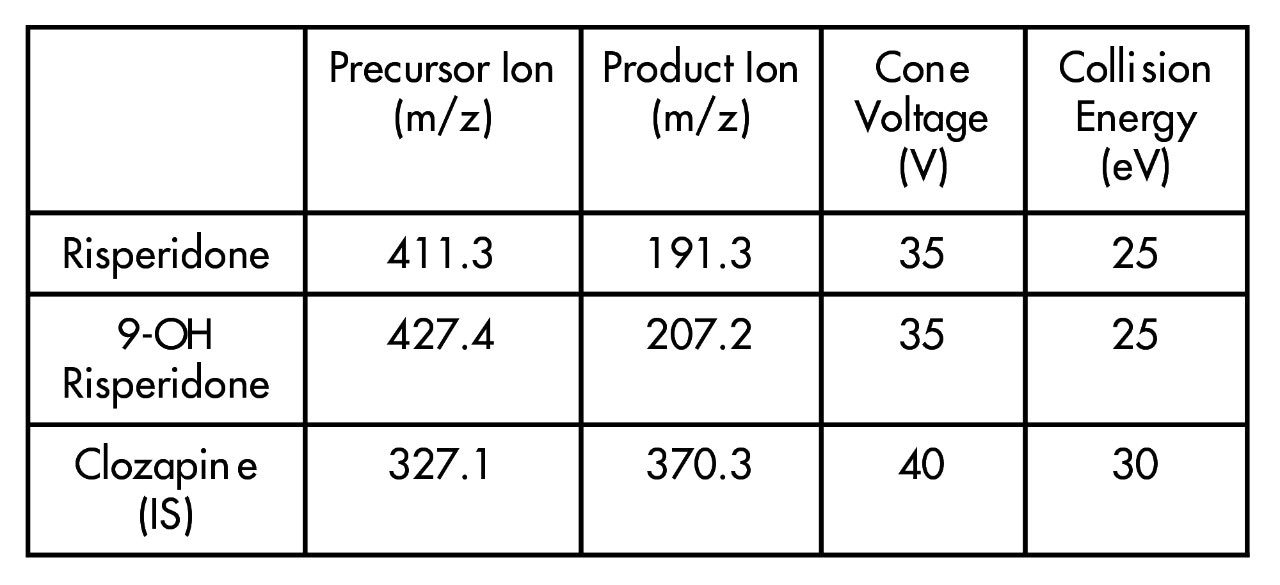
MRM transitions: |
|
|
Dwell time: |
30 ms for all transitions |
|
Inter-scan delay: |
10 ms for all transitions |
Stock solutions (1 mg/mL) of risperidone, 9-hydroxyrisperidone and clozapine were prepared by dissolving the appropriate amount of compound in methanol. Working solutions containing both risperidone and 9-hydroxyrisperidone were then prepared by diluting the appropriate volume of stock solution with 50:50 v/v methanol:water to give the following concentrations:
0.2, 0.4, 1.0, 2.0,10, 40, 200, 320, and 400 ng/mL.
0.002, 0.006, 1.4, 3.0, and 4.0 μg/mL.
An appropriate volume of clozapine was diluted with 50:50 v/v methanol: water to give a internal standard working solution of 0.5 μg/mL.
All solutions were stored at 4 °C for 1 month.
A calibration curve for the determination of the risperidone and 9-hydroxyrisperidone in human plasma was prepared fresh on the day of analysis at the following concentrations:
0.1, 0.2, 0.5, 1.0, 5.0, 20, 100, 160, and 200 ng/mL.
Additionally, bulk plasma QC samples were prepared at the following concentrations:
0.1, 0.3, 70, 150, and 200 ng/mL.
Bulk QC samples were stored at –20 °C until required.
Samples were prepared by taking 100 μL of spiked plasma and adding 50 μL of internal standard working solution (0.5 μg/mL). A 1 mL aliquot of water was then added to dilute the sample.
SPE using the Waters Oasis MCX μElution Plate, 30 μm particle, 96-well, Part Number 186001830BA
Calibration curves were generated using the Waters QuanLynx Application Manager for MassLynx Software by plotting the peak area ratio of Risperidone or 9-hydroxyrisperidone to the internal standard for each calibration concentration (Figures 2 and 3). The linear regression was constructed from 0.1–200 ng/mL (excluding the origin) and a weighting of 1/x2 was applied. For all validation batches, the calibration curve was analyzed in duplicate. All of the calibration curves generated had an r2 > 0.996 and all calibration points were within ±15% of their theoretical concentration.


The sensitivity (LLOQ) of the assay was confirmed to be 0.1 ng/mL, and was assessed by the accuracy and precision of six replicate LLOQ QC samples (Figure 4). Inter-batch accuracy and precision were 99.8% and 8.9%, respectively, for risperidone, and 95% and 5.5%, respectively, for 9-hydroxyrisperidone.

Intra-batch accuracy and precision was assessed by the analysis of six replicates at each QC level within each validation batch.
Inter-batch accuracy and precision was assessed by the analysis of each QC level (six replicates) within three validation batches.
The intra- and inter-batch accuracy and precision for both risperidone (Figure 5) and 9-hydroxyrisperidone (Figure 6) exceeds the FDA requirements for method validation.


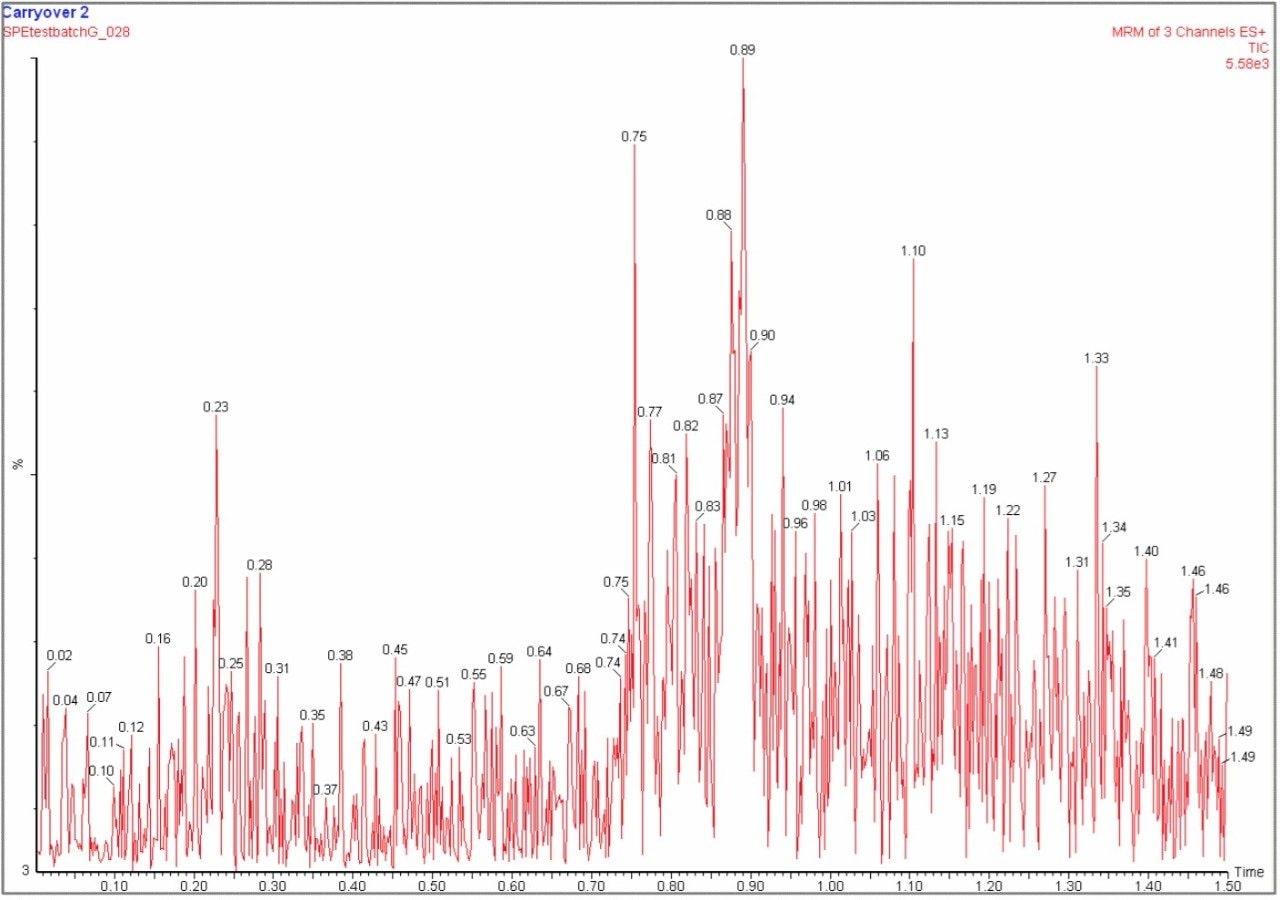
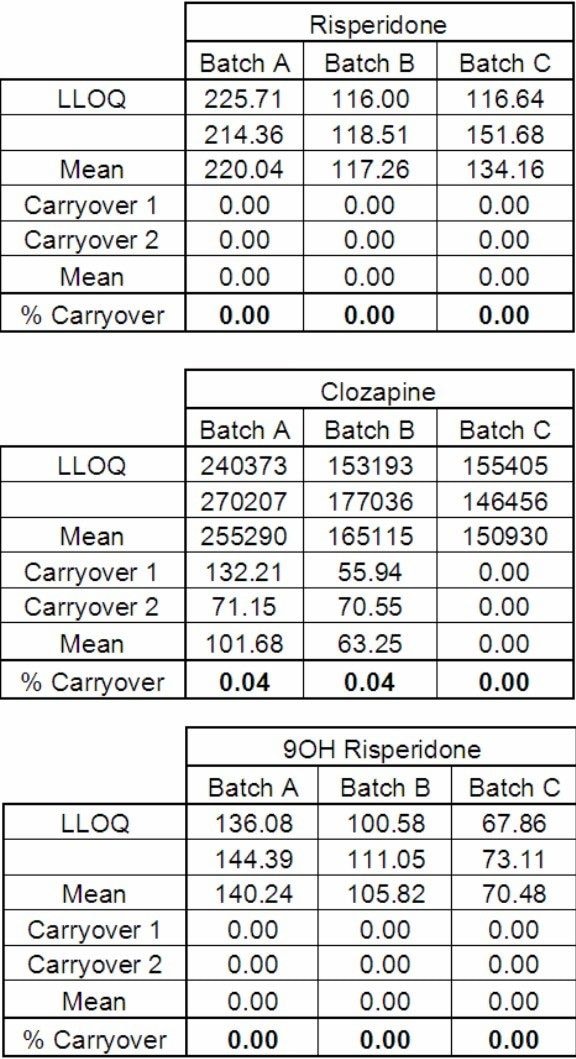
The carryover for the UPLC method was assessed by the duplicate injections of extracted plasma blanks directly after a high concentration (200 ng/mL) standard (Figure 7). The results showed that there was no detectable carryover of risperidone and 9-hydroxyrisperidone, and negligible levels of clozapine were observed in some of the samples (< 0.04% carryover) (Figure 8).
The selectivity of the method was assed by the analysis of blank plasma extracts from six different, non-pooled, human plasma sources (Figure 9).

After analysis, all six sources of plasma tested had no risperidone, 9-hydorxyrisperidone, or clozapine peaks present.
The following stability experiments were carried out:
– 24 hour extract stability, 4 °C
– Freeze/thaw stability, –20 °C
– Room temperature matrix stability
All stability experiments passed the relevant FDA guidelines.* Plasma samples and plasma extracts were stable for 24 hours at room temperature and at 4 °C, respectively. Plasma samples were stable for three freeze/thaw cycles at –20 °C.
*Raw data available upon request
Recovery of risperidone, 9-hydroxyrisperidone, and clozapine was assessed by the comparison of peak areas of extracted low-, mid- and high-concentration QC samples (0.3, 70, and 160 ng/mL, respectively) to post-spiked plasma extracts at the same concentrations, assuming 100% recovery (Figure 10). All recoveries were found to be >90% across the validation range.

Ion suppression is generally caused by endogenous matrix components that are not removed during sample preparation, and co-elute with the peaks of interest and compete for ionization in the ion source.
Ion suppression was assessed from six individual sources of human plasma both qualitatively (using a T-infusion experiment, Figure 11) and qualitatively (by comparison of post-spiked plasma extracts at the LLOQ (0.1 ng/mL) to post-spiked solutions containing no matrix components, Figure 12).


Results from the qualitative matrix effect tests showed no signs of a matrix effect and was confirmed by the quantitative tests that showed negligible levels of ion suppression.
A UPLC-MS/MS method has been validated for the determination of risperidone and 9-hydroxyrisperidone concentrations in human plasma, meeting the FDA Guidance for Industry for Bioanalytical Method Validation. The combination of the Oasis MCX μElution plate for easy sample preparation with the powerful ACQUITY UPLC/Quattro Premier XE platform allowed a fast and robust method to be developed. The sample preparation effectively removed all matrix components from the plasma extract that could lead to ion suppression. Low UPLC system volumes and innovative 1.7 μm particles enabled fast LC method development and, due to the narrow peaks produced and the resulting increase in signal-to-noise, a small sample volume of 100 μL could be used.
720001444, December 2005