
For forensic toxicology use only.
In this application note we demonstrate, a UPLC-MS/MS method was developed to separate morphine and five of its metabolites on a 2.1 x 100 mm, 1.8 μm ACQUITY UPLC HSS T3 Column in a single run using an ACQUITY UPLC System connected to a fast-scanning triple quadrupole MS detector (TQD).
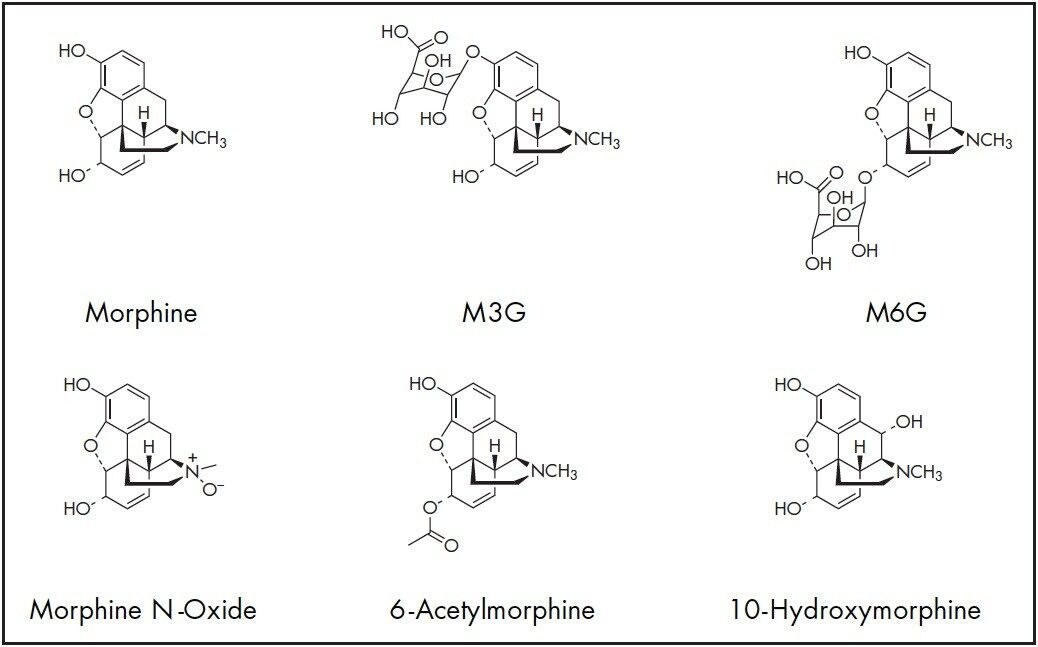
Morphine is an effective pain-relieving drug that is primarily metabolized into morphine-3-glucuronide (M3G) and morphine- 6-glucuronide (M6G). The highly potent M6G may have adverse effects such as respiratory depression and renal failure if accumulated in the body. Other metabolites of morphine include morphine N-oxide, 6-acetylmorphine and 10-hydroxymorphine. As morphine abuse continues to affect modern society, an effective method must be established to analyze morphine and its metabolites in biological fluid samples.1
In this work, a UPLC-MS/MS method was developed to separate morphine and five of its metabolites on a 2.1 x 100 mm, 1.8 μm ACQUITY UPLC HSS T3 Column in a single run using an ACQUITY UPLC System connected to a fast-scanning triplequadrupole MS detector (TQD). The method achieved adequate retention of these very polar compounds by reversed-phase (RP) chromatography in an 8-minute total run time. Mixed-mode solid-phase extraction (SPE) uses both reversedphase and ion-exchange mechanisms to separate analytes more selectively from matrix components. Therefore, sample preparation of porcine plasma was performed with the Oasis Mixed-mode Cation eXchange (MCX) μElution Plate. The strong cation-exchange sorbent was chosen because morphine, its five metabolites and, their internal standards are basic compounds (pKa of morphine = 9.85). The structures of the six analytes are shown in Figure 1. The Oasis MCX SPE procedure requires a high-pH elution step that is not suitable for 6-acetylmorphine due to degradation. To avoid compound degradation prior to UPLC-MS/MS analysis, a neutralizing collection step was employed.2 The SPE recovery and reproducibility of the method were determined, as well as the linearity and lower limit of quantitation (LLOQ) for each analyte.
|
System: |
Waters ACQUITY UPLC System |
|
Column: |
ACQUITY UPLC HSS T3 Column 2.1 x 100 mm, 1.8 μm |
|
Part Number: |
186003539 |
|
Column Temp: |
45 °C |
|
Sample Temp: |
4 °C |
|
Flow Rate: |
0.5 mL/min |
|
Mobile Phase A: |
0.1 % HCOOH in H2O |
|
Mobile Phase B: |
CH3OH |
|
Injection Volume: |
15 μL |
|
Injection Mode: |
Partial loop with needle overfill (PLNO; 20 μL loop size) |
|
Weak Needle Wash: |
95/5 (H2O/CH3OH) |
|
Strong Needle Wash: |
95/5 (CH3CN/H2O) |
|
Time(min) |
Profile |
Curve |
|
|
A (%) |
B (%) |
||
|
Initial |
98.0 |
2.0 |
- |
|
3.0 |
40.0 |
60.0 |
6 |
|
3.5 |
2.0 |
98.0 |
6 |
|
5.0 |
2.0 |
98.0 |
6 |
|
5.1 |
98.0 |
2.0 |
6 |
|
8.0 |
98.0 |
2.0 |
6 |
|
MS System: |
Waters ACQUITY TQD |
|
Ionization Mode: |
Electrospray Positive |
|
Capillary Voltage: |
1.0 kV |
|
Cone Gas: |
50 L/Hr |
|
Desolvation Gas: |
800 L/Hr |
|
Collision Cell Pressure: |
4.07e -3 mbar |
|
Desolvation Temp: |
350 °C |
|
Source Temp: |
120 °C |
|
Dwell Time: |
10 ms |
|
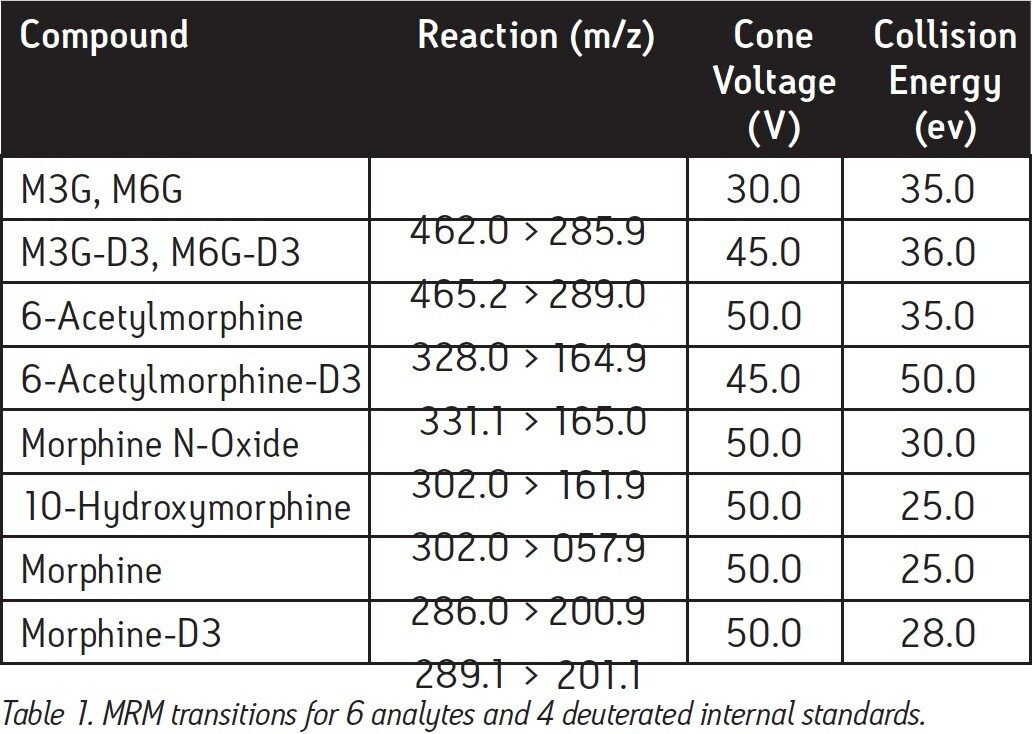
MRM Transitions: |
See Table 1 |
All of the six standard and four internal standard (IS) solutions were received at a concentration of 100 μg/mL from the manufacturer Working solutions were prepared by diluting and mixing each of the six standard solutions with 50:50 CH3OH:H2O (v:v) to give the appropriate concentrations for calibration. The four individual IS solutions were diluted with 50:50 CH3OH:H2O (v:v) to give an IS working solution of 1 μg/mL for each compound.
Calibration curves for each of the six compounds in porcine plasma were generated with individual points at the following concentrations: 0.1, 0.25, 0.5, 1, 5, 10, 25, 50, 100, and 250 ng/mL. The internal standard working solution was spiked to a final concentration of 25 ng/mL each. Porcine plasma was spiked as follows for each concentration:
50 μL standard working solution and 50 μL internal standard working solution were added to 2 mL porcine plasma. The sample was then vortexed and diluted with 1.9 mL 4 % H3PO4 in water. These solutions are the pretreated (diluted and acidified) plasma solutions which are ready for SPE. The plasma blank was prepared by adding 50 μL of 50:50 CH3OH:H2O (v:v) and 50 μL internal standard working solution to 2 mL porcine plasma and 1.9 mL 4 % H3PO4 in water.
Solid-phase extraction was performed with the Oasis MCX μElution 96-well Plate. Because acetylmorphine was found to be unstable at high pH, the eluates were collected into a collection plate containing 3 % HCOOH in CH3OH in order to neutralize the basic elution solvent (final pH ~ 4.5). These samples were then evaporated and reconstituted with water to ensure compatibility with the starting gradient conditions.
The detailed SPE procedure is described below:
Oasis MCX μElution 96-well plate (Part Number: 186001830BA)
Recovery for the analytes and internal standards was determined by comparing the MRM peak areas of pre-extracted spiked samples at 25 ng/mL to those of the post-extracted spiked samples at the same concentration. The pre-extracted samples were spiked with 50 μL of the standard mixture at 1 μg/mL each and extracted in the plate. Blank plasma samples were extracted in the plate and then spiked with 10 μL of the standard mixture at 625 ng/mL each. The pre-extracted and post-extracted spiked samples were evaporated to dryness and reconstituted with 50 μL water. Extraction was performed on three different days (N = 4 each day).
Calibration curves were generated using the Waters QuanLynx Application Manager for MassLynx MS Software by plotting the ratio of analyte to internal standard peak area for each concentration. The linear regression was constructed using a weighting of 1/x with the origin excluded. The percentage deviation for each calibration point was obtained from QuanLynx by comparing the concentration value calculated from the linear regression to the expected value.
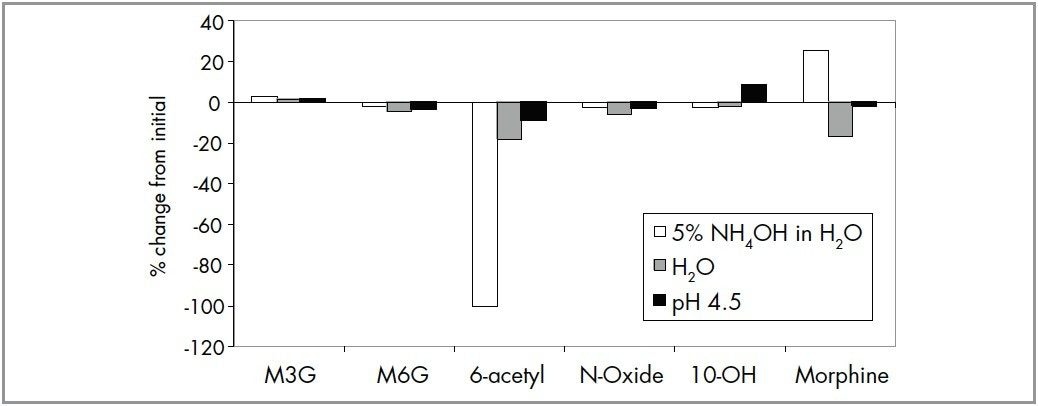
To investigate analyte stability, a standard mixture containing 20 ng/mL of each analyte was prepared in H2O, 5% NH4OH in H2O, 5% NH4OH:3% formic acid (1:1, v:v) and stored at 4 °C (set temperature of the autosampler) for 24 hours. Stability of the analytes was evaluated by comparing the area counts after 24 hours to the area counts obtained immediately after the solutions were prepared. In general, the compounds are stable in H2O. At high pH, however, 6-acetylmorphine degrades completely after 24 hrs, and area counts for morphine increase by 25% (Figure 2). It is expected that some of the 6-acetylmorphine hydrolyzes to morphine at high pH. All the six compounds were stable (<10 % change) in the neutralizing solution.
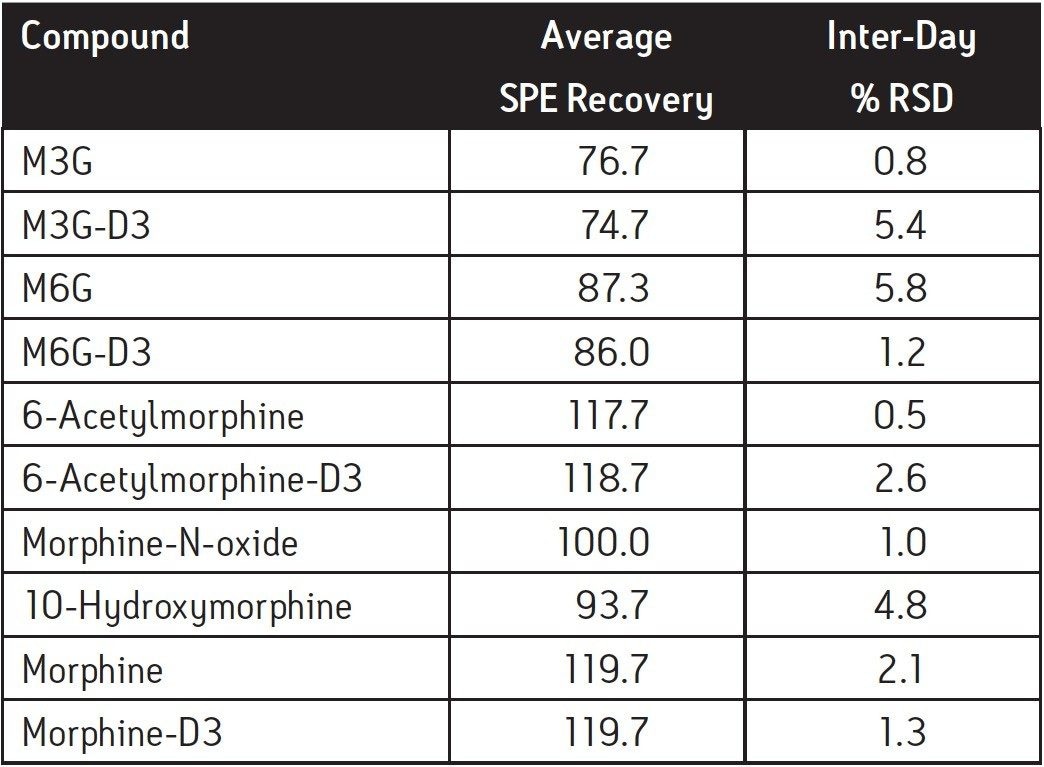
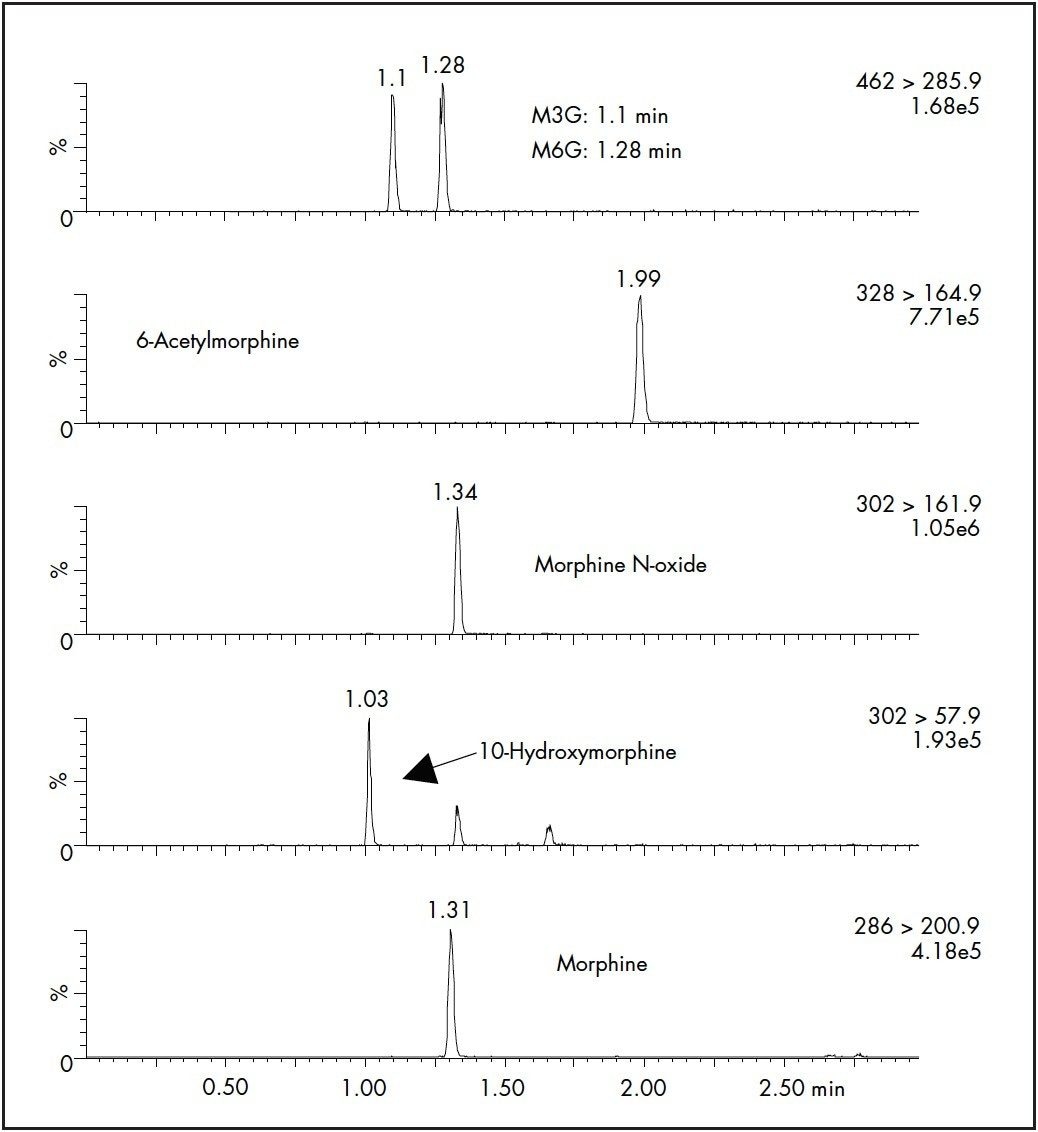
SPE recoveries for most of the compounds at 25 ng/mL were >90% except for the glucuronide metabolites, that had slightly lower recovery (Table 2). Inter-day SPE reproducibility was better than 6%. A representative chromatogram of a pre-extracted spiked sample is shown in Figure 3.
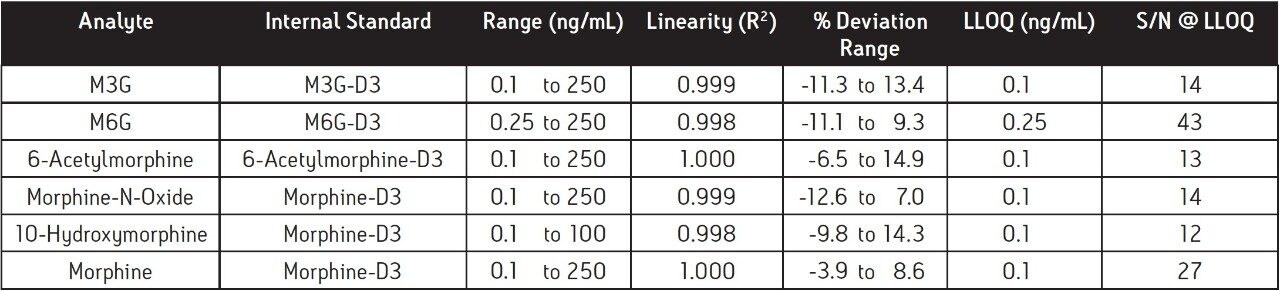
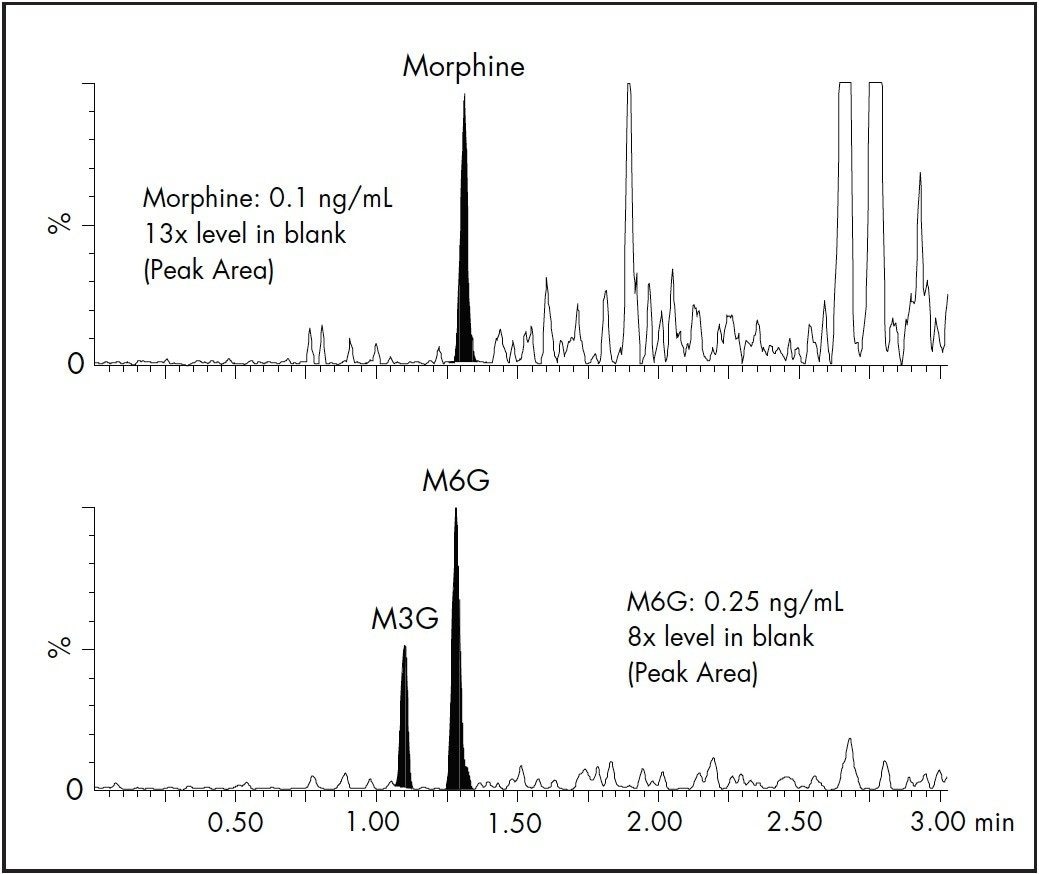
The results for linearity and LLOQ are shown in Table 3. All calibration curves had an R2 ≥0.998, and % deviation for each point was < ± 15 % of the expected values. The LLOQ values (5x level in blank 4) for all analytes were determined to be 0.1 or 0.25 ng/mL. The signal-to-noise (S/N) ratio of each analyte at the LLOQ was also determined. Two representative chromatograms showing the LLOQ for morphine and M6G are shown in Figure 4.
A method for the simultaneous extraction and quantitation of morphine and five of its metabolites in porcine plasma was developed. All six compounds were analyzed in a single UPLC-MS/MS run in 8 minutes. A neutralizing collection step was used in the SPE protocol to prevent analyte degradation. The SPE procedure using an Oasis MCX μElution Plate was able to achieve consistent recoveries ranging from 77% to 120%, depending on the analyte. The method was linear over at least 3 orders of magnitude with R2 ≥0.998, and achieved LLOQ values in the range of 0.1 to 0.25 ng/mL. This method achieves the desired detection limits in less time than previously published methods, and addresses the issue of analyte instability for the selective extraction of polar compounds.
720002896, January 2009