This is an Application Brief and does not contain a detailed Experimental section.
This technology brief assess the performance of the ACQUITY UPLC I-Class PLUS System coupled with the Xevo TQ-S Mass Spectrometer for the analysis of pharmaceutical drugs used in CYP inhibition assays compared with the ACQUITY UPLC I-Class System.
The ACQUITY UPLC I-Class PLUS System produced results comparable with the ACQUITY UPLC I-Class System for the analysis of pharmaceutical drugs. Retention time reproducibility was excellent with RSD below 0.5%. The ACQUITY UPLC I-Class PLUS System gives confident and reliable results to aid investigators in establishing DDI and CYP experiments in the process of evaluating new drugs.
The ACQUITY UPLC I-Class PLUS System with the Xevo TQ-S Mass Spectrometer provides reproducible and reliable results for the analysis of pharmaceutical drugs.
Polypharmacy (use of multiple concurrent prescription drugs) in US adults is increasing with significant prevalence in elderly populations treating multiple disease states.1,2 As the number of prescriptions increase for an individual, there is an increase for the risk of drug-drug interactions (DDI) primarily due to induction or inhibition of Cytochrome P450s (CYP) with these interactions leading to potentially dangerous side effects. CYP is a superfamily of enzymes that are, among others, responsible for the metabolic transformation of drug substances.³ For this reason, it has become a requirement to include CYP inhibition assays to assess DDIs in new drug development.4,5 Libraries of compounds with known metabolic fates and the investigational drug are incubated with liver microsomes or hepatocytes and after a time the sample is analyzed.6 The data obtained gives a snapshot of the potential clinical consequences and gives investigators a framework for future studies.
In this work, we assess the performance of the ACQUITY UPLC I-Class PLUS System coupled with the Xevo TQ-S Mass Spectrometer for the analysis of pharmaceutical drugs used in CYP inhibition assays compared with the ACQUITY UPLC I-Class System.
Compound libraries were purchased from Enzo Life Sciences (Farmingdale, NY, USA). The standards were supplied in DMSO (96-well plate format) and were diluted to 1 µM in 50:50 acetonitrile/water (v/v) for MRM optimization and 25 nM in 95:5 water/acetonitrile (v/v) for UPLC analysis. MRM optimization was performed using the MassLynx v4.1 application manager QuanOptimize. A UPLC separation was achieved on a CORTECS C18+, 1.6 µm, 2.1 x 100 mm Column at a flow rate of 0.5 mL/min and gradient from 5% B to 95% B over 10 minutes, with a one-minute hold and re-equilibration for 10-column volumes at a total run time of 16 minutes. The mobile phase consisted of (A) water and (B) acetonitrile, each with 0.1% formic acid and injection volume was 5 µL. The retention times were measured using the TargetLynx Application Manager, and were the average of five-replicate injections.
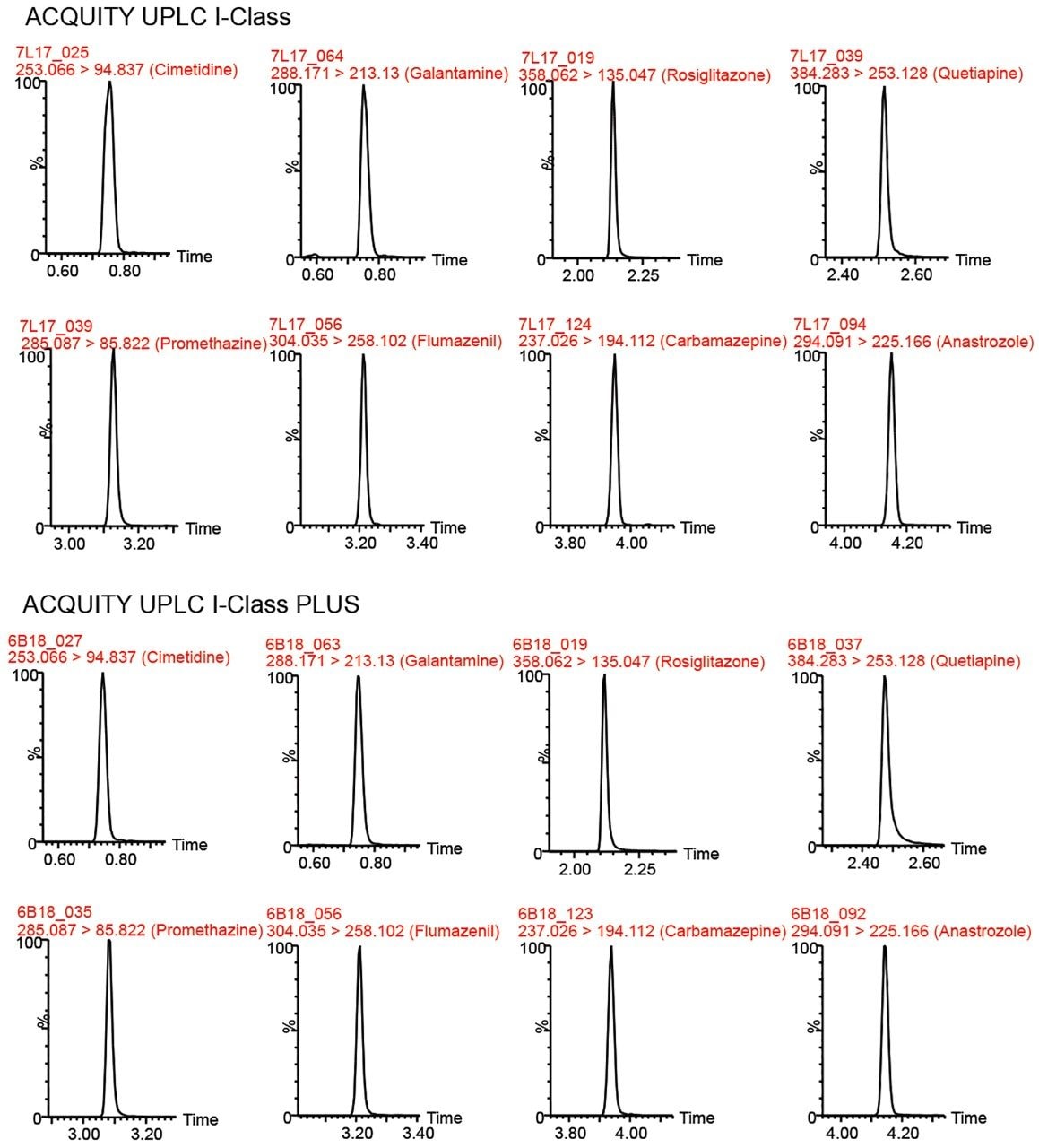
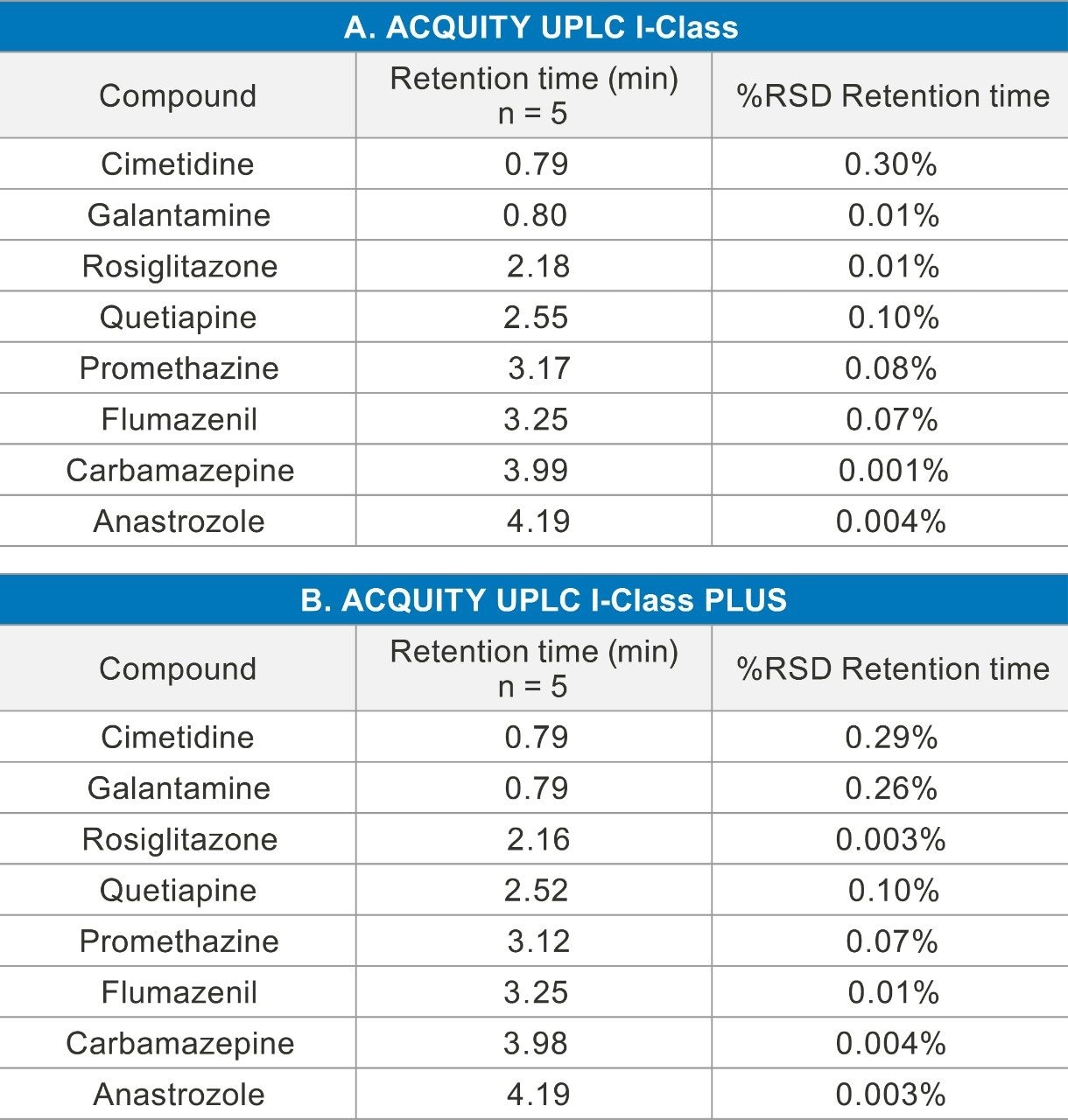
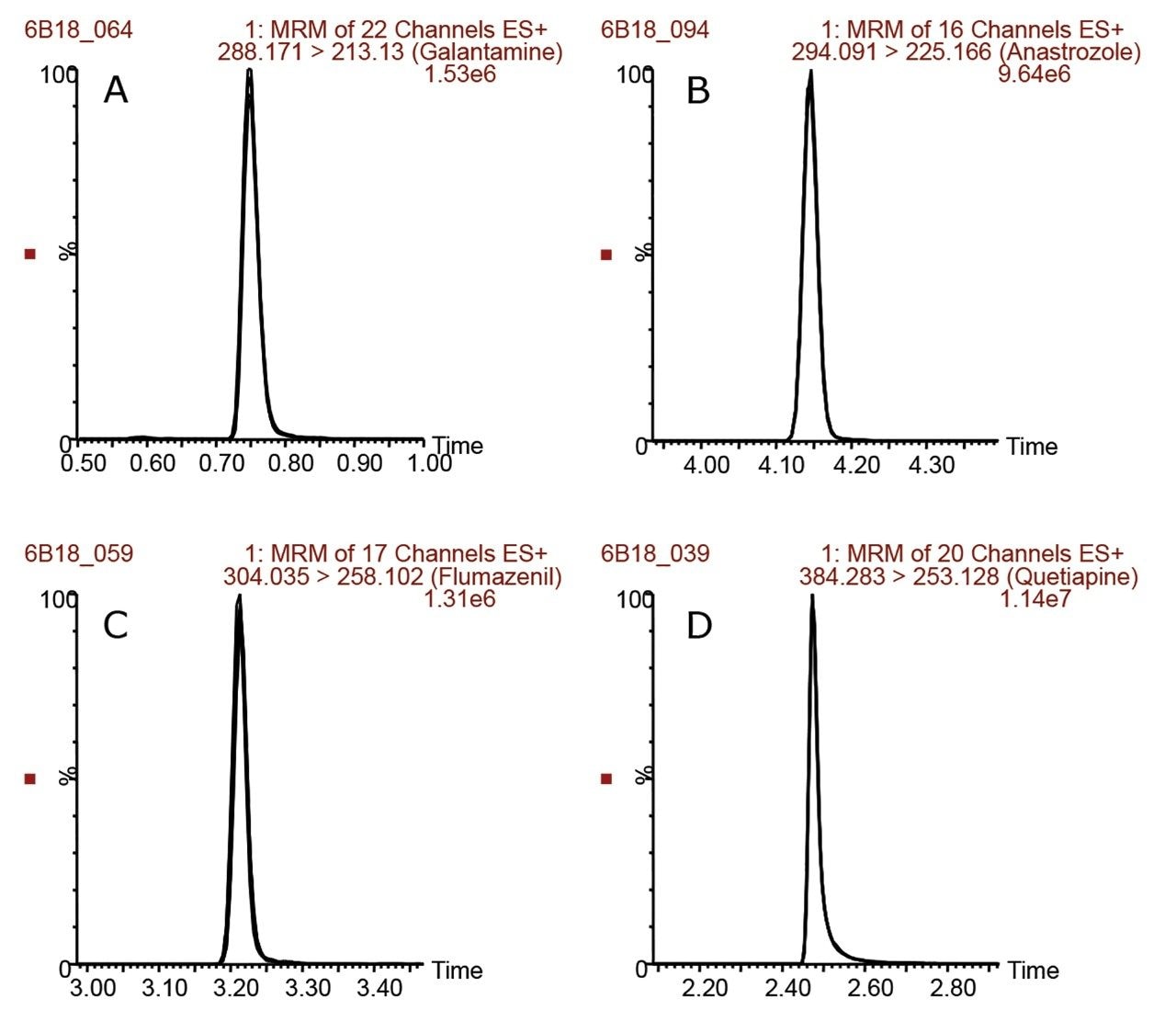
One hundred seven compounds commonly used in CYP inhibition assays were analyzed using the same column, mobile phase, and mass spectrometer by both the ACQUITY UPLC I-Class and the ACQUITY UPLC I-Class PLUS Systems. Figure 1 shows chromatograms for eight representative compounds on each LC system with tabulated results in Table 1. The retention times between the two systems were comparable for all the compounds tested. Retention time reproducibility is illustrated in Figure 2 for representative compounds, and was less than 0.6% and 0.5% RSD for the 107 compounds for the ACQUITY UPLC I-Class and ACQUITY UPLC I-Class PLUS Systems, respectively. These results demonstrate the reproducibility of the ACQUITY I-Class PLUS System and gives confidence in each analysis.
The ACQUITY UPLC I-Class PLUS System produced results comparable with the ACQUITY UPLC I-Class System for the analysis of pharmaceutical drugs. Retention time reproducibility was excellent with RSD below 0.5%. The ACQUITY UPLC I-Class PLUS System gives confident and reliable results to aid investigators in establishing DDI and CYP experiments in the process of evaluating new drugs.
720006252, April 2018