In this Application, we monitor the presence of pharmaceuticals in drinking water supplies and examining their long term effects on human health.
Many hundreds of active compounds are used in both human and veterinary drug formulations. Due to the many different applications related to pharmaceuticals, their residues can reach the environment in multiple ways including excretion and manufacturing discharge. These compounds are not completely eliminated via sewage treatment plants, thus they can reach surface and groundwater supplies. Recently, there has been increased interest in monitoring for the presence of pharmaceuticals in drinking water supplies and examining their long term effects on human health.
|
Instrument: |
Waters 2690 HPLC or Waters 2795 HPLC, Quattro Ultima MS/MS |
|
LC Column: |
Waters XTerra C18, 3.5 μm, 10.0 cm, 2.1 mm |
|
Ionizatoin: |
Electrospray Positive (ES+) |
|
Acquisition: |
MRM mode, unit resolution |
|
Injection Volume: |
15 μL |
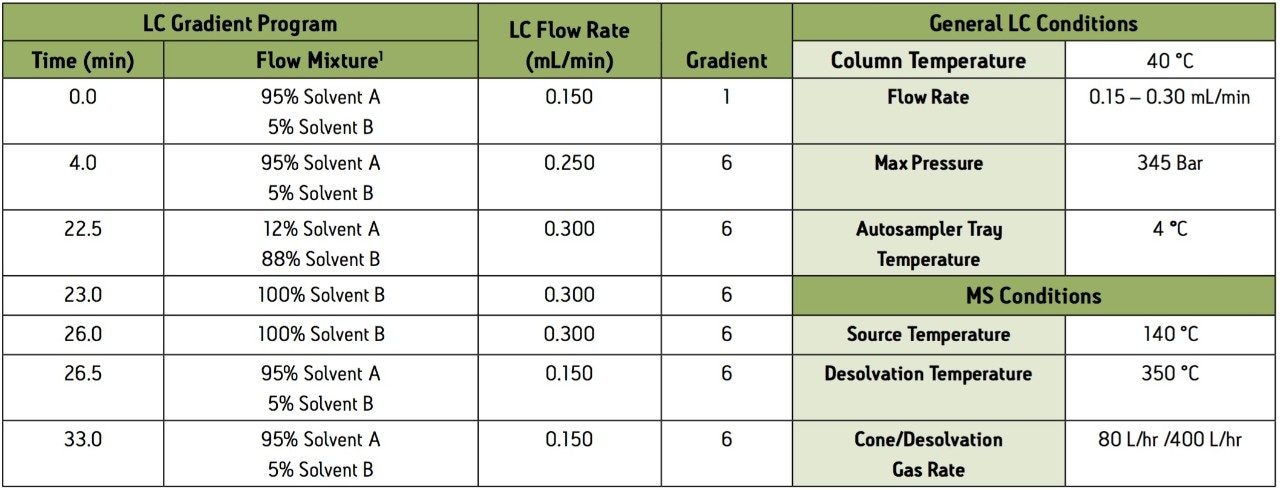
1 Solvent A = 0.3% Formic Acid and 0.1% Ammonium Formate in HPLC water
Solvent B = 1:1 Acetonitrile:Methanol
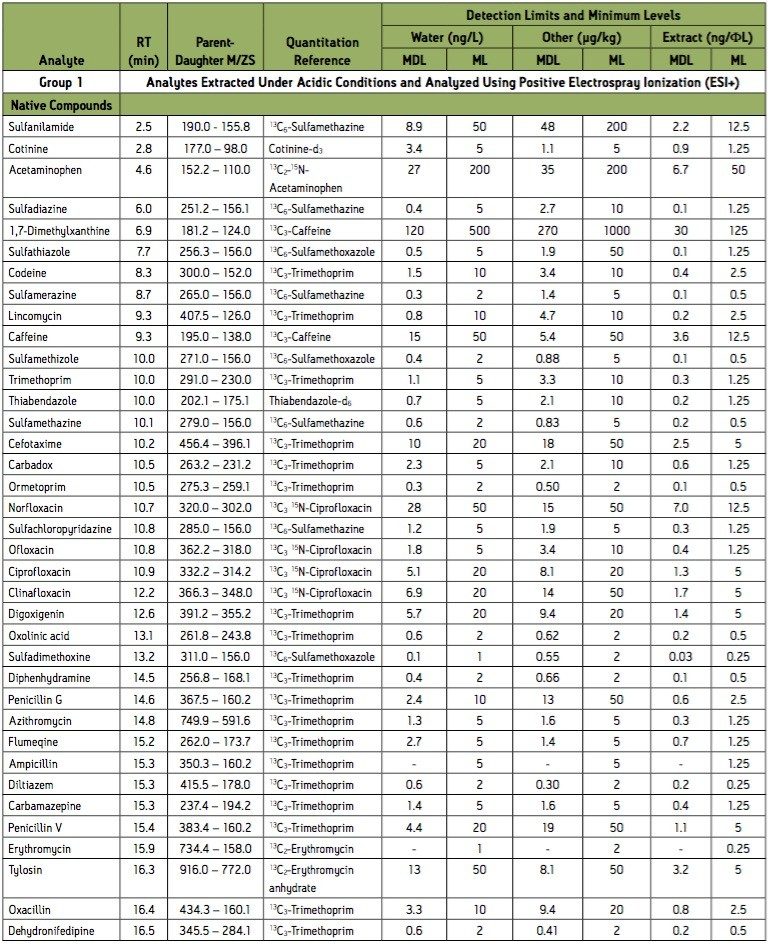
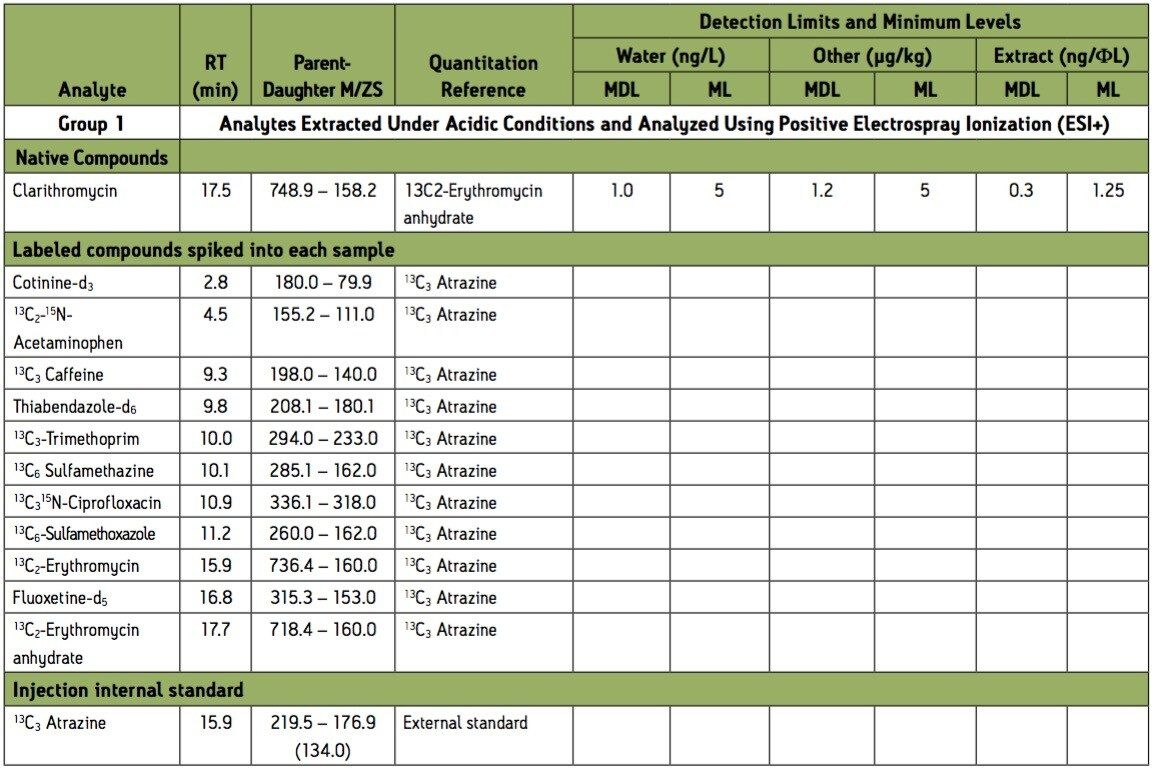
Group 1 – Acidic extraction, positive electrospray ionization (ESI+) instrument conditions.
|
Instrument: |
Waters 2690 HPLC or Waters 2795 HPLC, Quattro Ultima MS/MS |
|
LC Column: |
Waters XTerra C18, 3.5 μm, 10.0 cm, 2.1 mm |
|
Ionization: |
Negative Ion Electrospray |
|
Acquisition: |
MRM mode, unit resolution |
|
Injection Volume: |
5 μL |
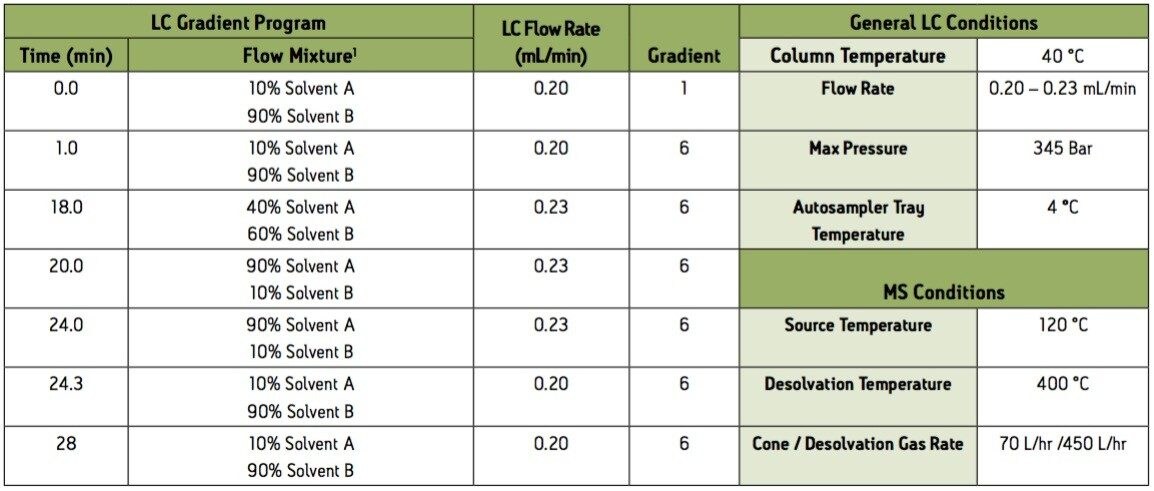
1 Solvent A = 1:1 acetonitrile:methanol, with 5 mM Oxalic Acid
Solvent B = HPLC H2O, with 5 mM Oxalic Acid.
Group 2 – Acidic extraction positive electrospray ionization (ESI+) instrument conditions.
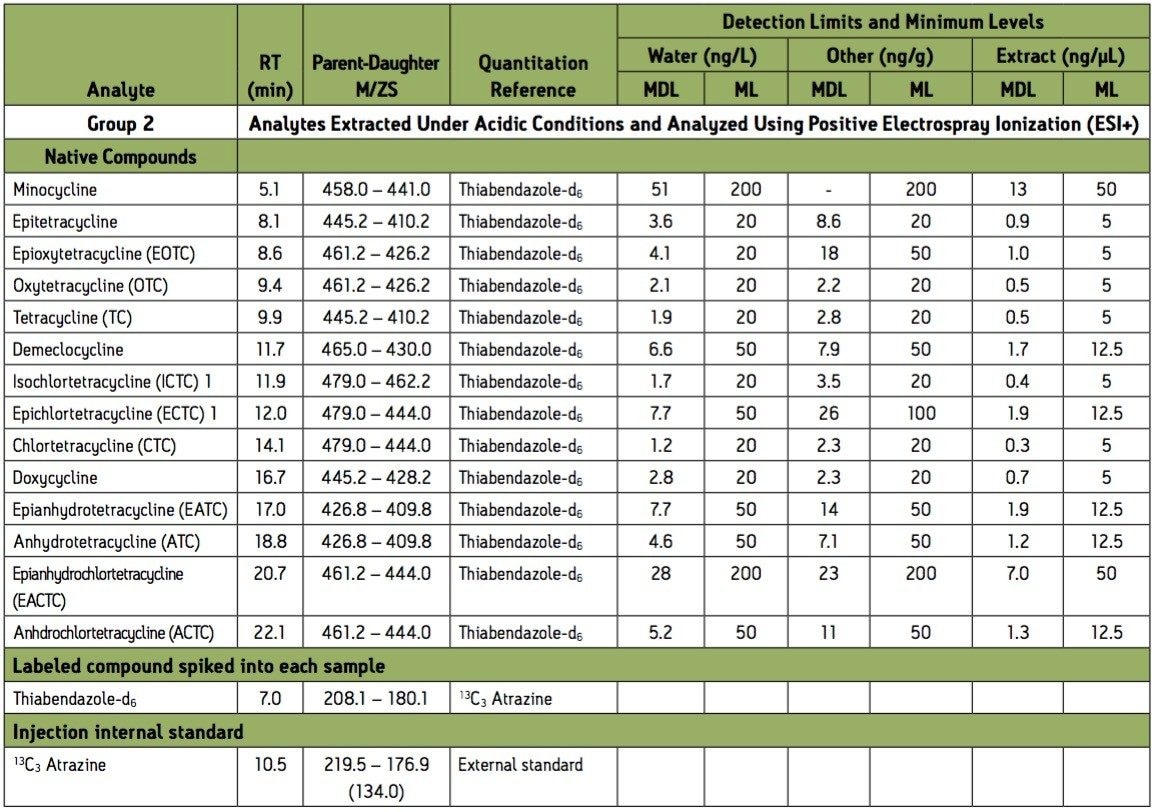
1 Isochlortetracycline (ICTC) is reported as the sum ICTC + ECTC due to a common transition ion.
Group 2 acidic extraction positive electrospray ionization (ESI+) compound retention times (RTs), parent-daughter transitions, quantitation references, method detection limits, and minimum levels of quantitation.
|
Instrument: |
Waters 2690 HPLC or Waters 2795 HPLC, Quattro Ultima MS/MS |
|
LC Column: |
Waters XTerra C18, 3.5 μm, 10.0 cm, 2.1 mm |
|
Ionization: |
Negative Ion Electrospray |
|
Acquisition: |
MRM mode, unit resolution |
|
Injection Volume: |
5 μL |
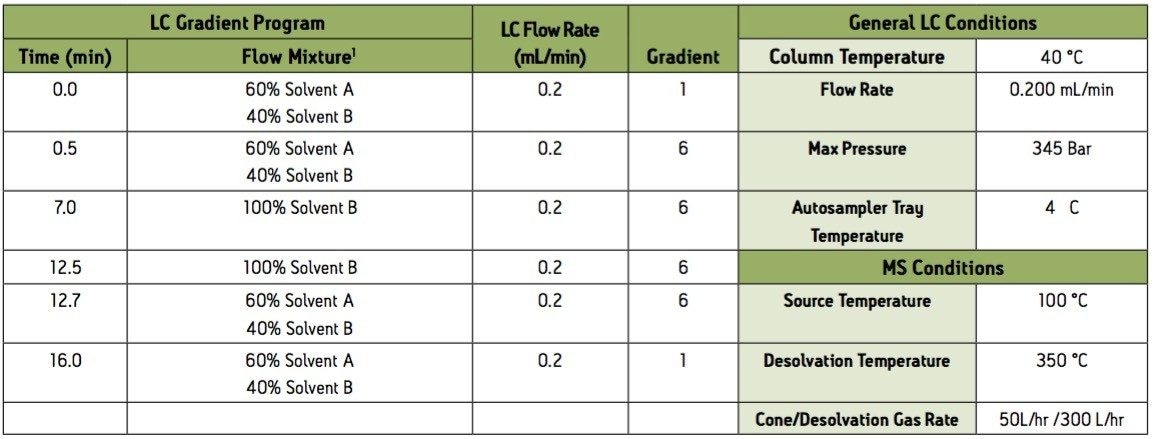
1 Solvent A = 0.1% Ammonium Acetate and 0.1% Acetic Acid in HPLC water
Solvent B = 1:1 MethanolAcetonitrile
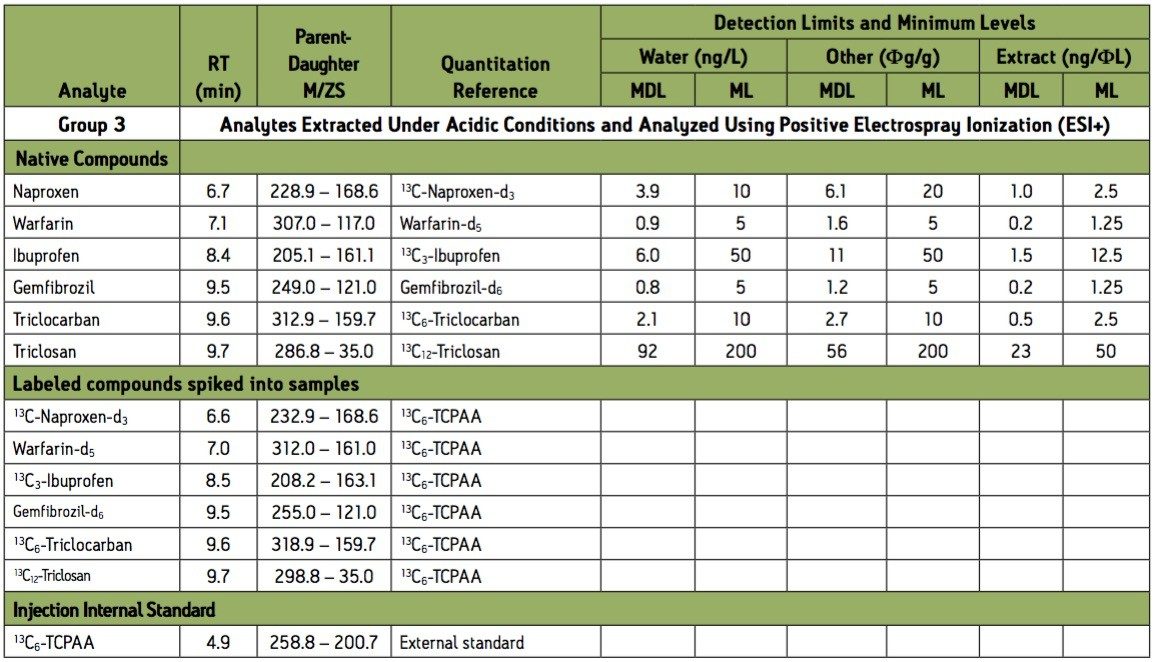
Group 3 Acidic extraction negative electrospray ionization (ESI-) instrument conditions.
|
Instrument: |
Waters 2690 HPLC or Waters 2795 HPLC, Quattro Ultima MS/MS |
|
LC Column: |
Waters Atlantis HILIC, 3.0 μm, 2.1x 100 mm |
|
Ionization: |
Electrospray Positive (ES+) |
|
Acquisition: |
MRM mode, unit resolution |
|
Purge Solvent: |
100% CH3CN (changed from H2O) |
|
Injection Volume: |
2.0 μL |
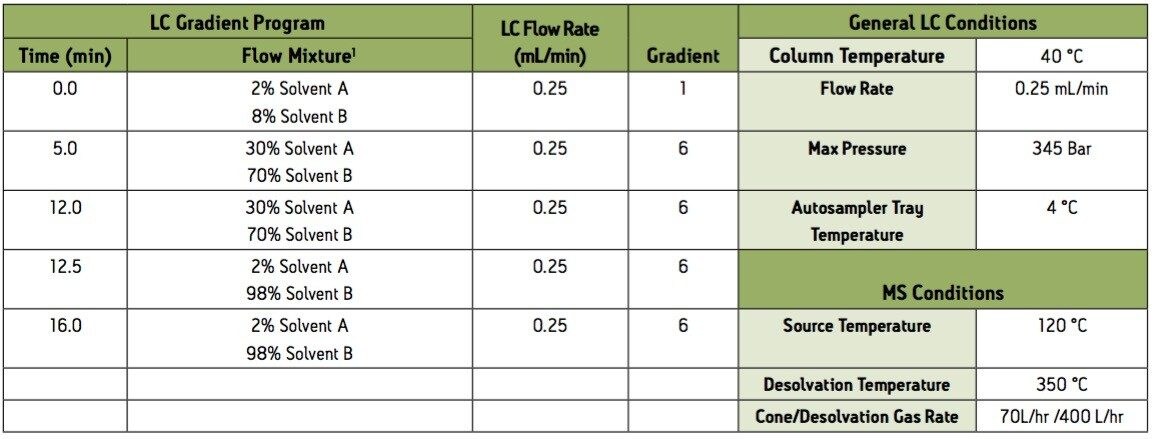
1 Solvent A = 0.1% Acetic Acid/Ammonium Acetate Buffer
Solvent B = Acetonitrile
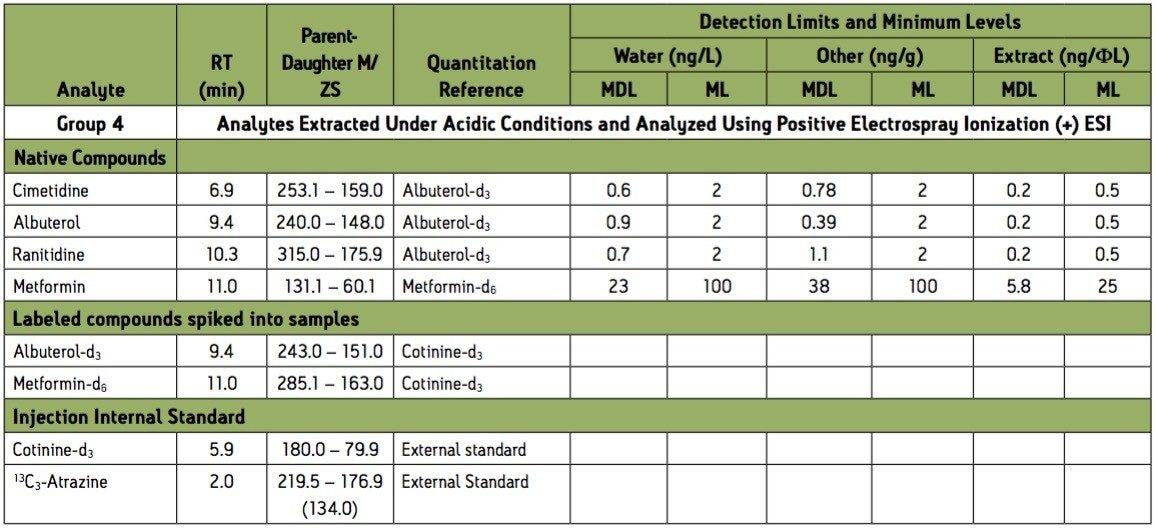
Group 4 – Basic extraction positive electrospray ionization (ESI+) instrument conditions.
720002734, August 2008