For forensic toxicology use only.
This is an Application Brief and does not contain a detailed Experimental section.
The use of hair as a biological matrix for forensic toxicology testing has increased in popularity over the last decade, with laboratories testing for a panel of drugs as recommended by societies such as the Society of Hair Testing (SoHT) or the European Workplace Drug Testing Society (EWDTS).1,2 A robust sample preparation workflow and sensitive UPLC-MS/MS method has been developed that allows the user to determine such panels of drugs of abuse in hair to meet these guidelines. The workflow allows the user to determine multiple classes of drugs, such as opiates, amphetamines, cocaine, ketamine, buprenorphine, benzodiazepines, and their metabolites, as well as tetrahydrocannabinol (THC) and its metabolite carboxy-THC (cTHC), from a single hair specimen. Following incubation in a commercially available extraction buffer, the analytes were separated from the remaining hair matrix using a single solid phase extraction (SPE) method using OASIS PRiME MCX 96-Well Plates. Two ACQUITY UPLC I-Class System/Xevo TQ-S micro System LC-MS/MS methods were developed; one to measure 29 “basic drugs” and the second to measure THC, cannabidiol, and cannabinol. A different sample preparation method, using the remaining incubation mixture, along with a new LC-MS/MS method are required to measure carboxy-THC to confirm the presence of THC.3 This workflow allows all the analytes to be quantified at concentrations below the recommended confirmation cut-offs of the two societies, in under 10 minutes per sample, allowing for a large number of samples to be analyzed in a short period of time.
Previously, we have discussed the benefits of hair as a biological matrix for forensic toxicology testing and have described a highly sensitive method for carboxy-THC, a metabolite of THC that can be used to definitively prove active use of cannabis.3 The procedure utilized 20 mg of hair and extraction of drugs from the hair matrix was achieved using M3 Reagent followed by sample clean-up using OASIS PRiME HLB and analysis using an ACQUITY UPLC I-Class System/Xevo TQ-S micro Mass Spectrometer LC-MS/MS method.
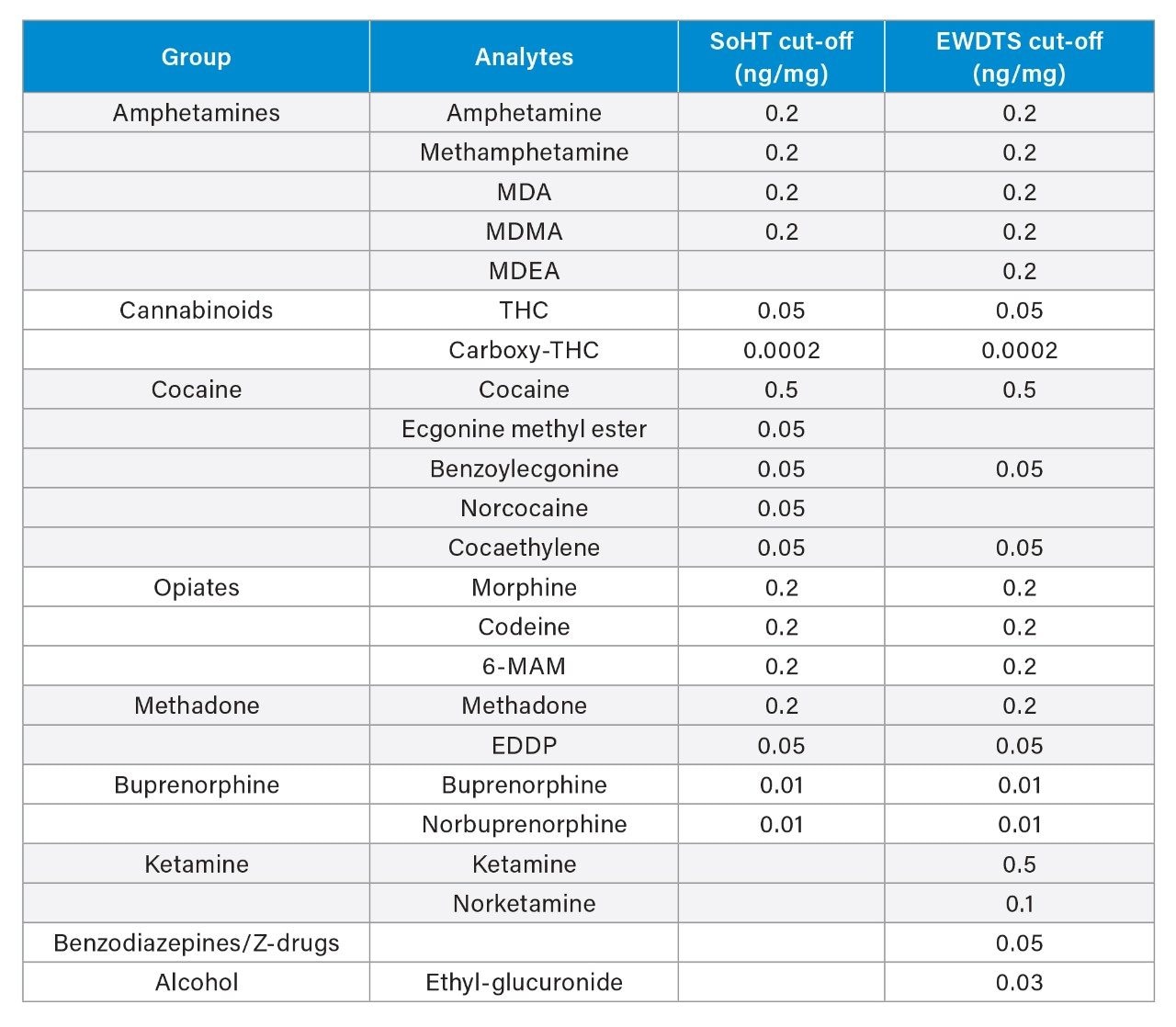
In routine practice, however, cTHC is only one compound from a large panel of drugs that need to be analyzed routinely and are included in recommended guidelines by societies such as the SoHT or EWDTS (see Table 1). The larger panel also includes opiates, amphetamines, cocaine, ketamine, buprenorphine, benzodiazepines, and their metabolites as well as tetrahydrocannabinol (THC). The recommended cut-offs for each drug class are different, and for some analytes are very low. Consequently, some laboratories apply several different procedures to ensure coverage of all the analytes in this panel. In practice, this may involve the use of differing extraction procedures, separate sample clean-up protocols, and even differing subsequent analytical methods to be applied on the same specimen to ensure appropriate sensitivity for the different drug classes. In addition to the inconvenience of applying multiple protocols, this potentially increases the amount of sample required, which can be problematic as the amount of hair available is usually limited. Thus, a more streamlined approach is required; a simplified sample preparation workflow that can improve laboratory efficiency and allow these analytes to be quantified in hair by a fast, robust, and sensitive analytical method is preferable. The workflow must allow for a large volume of tests to be carried out, while conforming to the guidelines recommended by societies such as the SoHT and the EWDTS.
Control hair was collected from volunteers and, following successive decontamination with dichloromethane, methanol, and diethyl ether, it was scissor-minced into 1–2 mm segments. M3 Reagent was supplied by Comedical, Trento, Italy, http://www.comedical.biz/.
Control hair (10–20 mg) was weighed into a glass centrifuge tube with a sealed cap and spiked with a panel of drugs of abuse at concentrations ranging from 0.5x cut-off to 200x cut-off. An internal standard (ISTD) mixture was added at 1 ng/mg along with M3 Reagent. The samples were heated for 60 min at 100 °C in an incubator and, once cooled, 100 µL of the sample was diluted with phosphoric acid before loading onto an OASIS PRiME MCX 30 mg 96-Well Plate (p/n: 186008916). The sample was washed with 2% formic acid followed by 50% methanol. Analytes were eluted with acetonitrile/methanol (9:1 v/v) containing ammonia. A 25-µL aliquot of the SPE eluant was taken for analysis of the “basic drugs” and a second 25-µL aliquot was taken for the analysis of THC.
The ACQUITY UPLC I-Class System with FTN (flow-through needle) was fitted with a 30-µL needle, which allowed 15 µL of sample to be analyzed. The two reconstituted samples were separated using an ammonium formate/acetonitrile gradient on an ACQUITY UPLC BEH C18 Column (p/n: 186002350) by two different inlet methods but the same mobile phases. Two MRM transitions were monitored for each analyte along with an MRM transition for each ISTD. The combined runtime of the two LC-MS/MS methods was 10 mins.
A chromatogram showing the separation of the “basic drugs” spiked into 10 mg of hair at the confirmation cut-off recommended by the SoHT and EWDTS is provided in Figure 1 and shows the quantifier MRM trace for 29 analytes. In this panel, the recommended cut-off levels for buprenorphine and norbuprenorphine are particularly challenging. Figure 2 shows the calibration curve and residuals plot for buprenorphine spiked into 20 mg of hair at concentrations ranging from 0.005 ng/mg to 2 ng/mg.
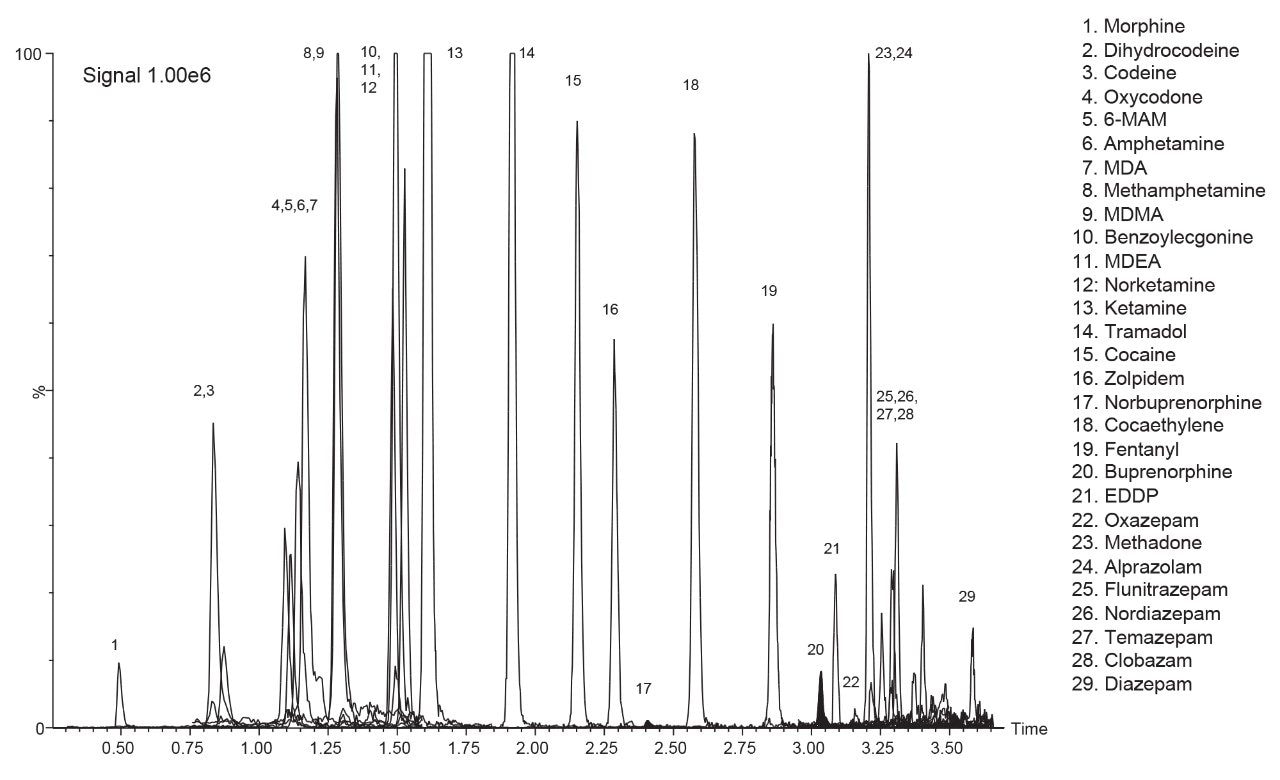
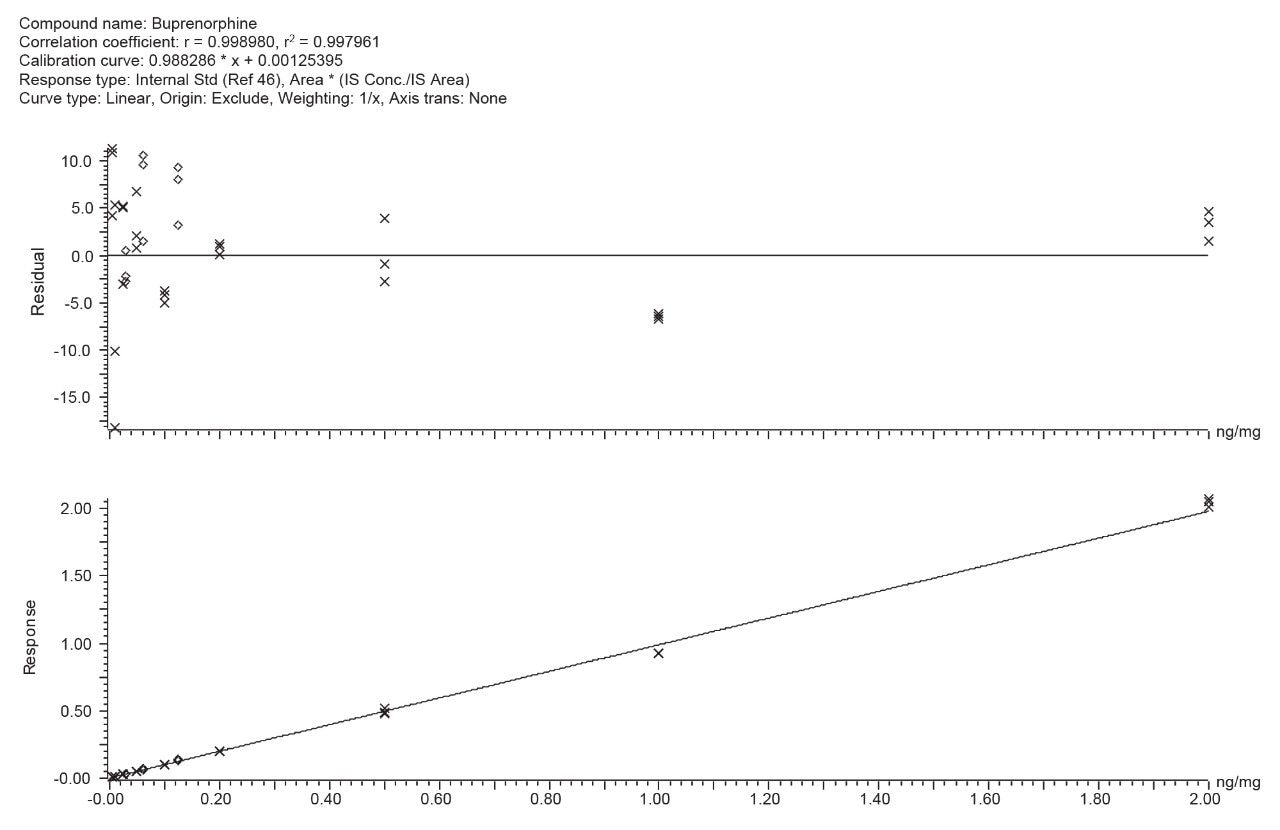
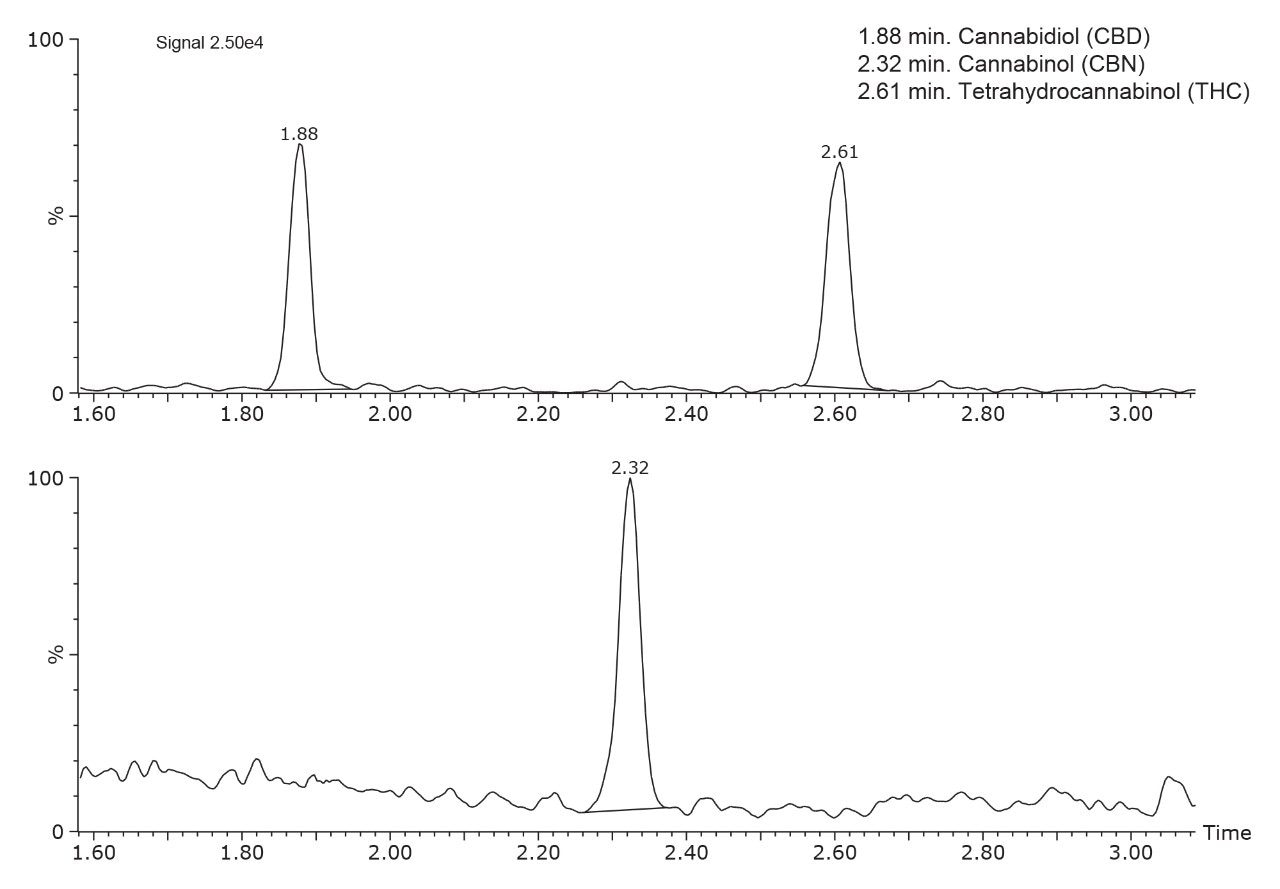
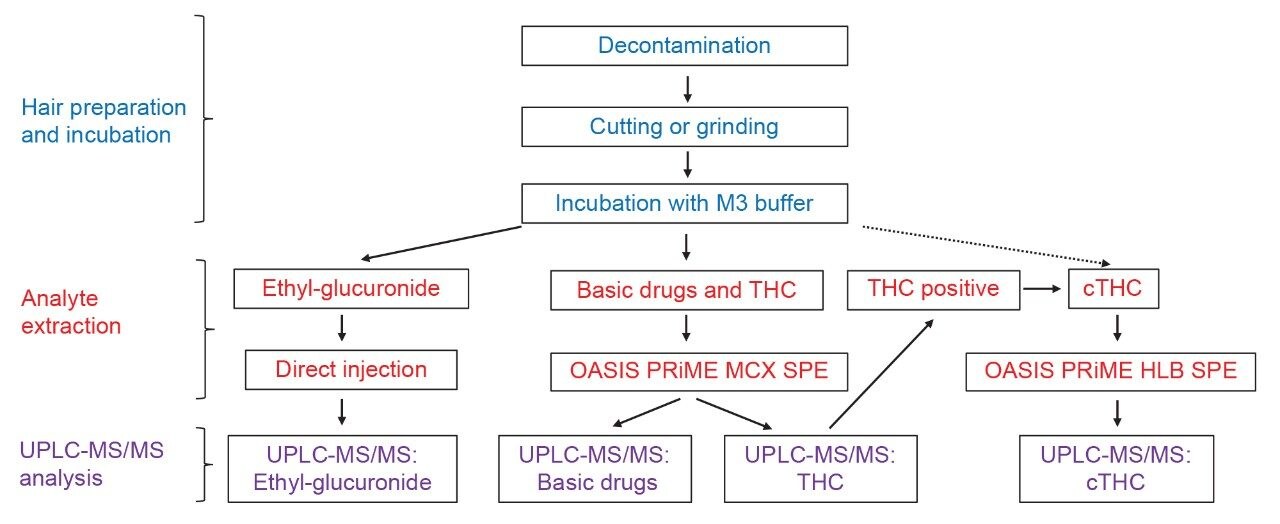
Figure 3 shows the quantifier MRM traces for THC, cannabidiol, and cannabinol spiked into hair at 0.05 ng/mg. To confirm the presence of THC in a sample, the remaining incubation mixture was used to detect cTHC by a separate analytical method as reported previously. The alcohol biomarker ethyl glucuronide can be measured from the M3 incubation mixture following the method described by Joya, et al.4 A potential complete workflow schematic is shown in Figure 4.
The increased use of hair for drug testing has highlighted the need for quick, accurate, reliable, and robust methods to quantify drugs of abuse at very low concentrations. This note details a workflow that can be used to quantify a panel of drugs at cut-off concentrations recommended by both the SoHT and EWDTS from a single hair specimen using a commercially available extraction buffer and OASIS PRiME MCX 96-Well Plate. In combination with the ACQUITY UPLC I-Class System and Xevo TQ-S micro Mass Spectrometer, it is possible to quantify a large number of compounds at low concentrations, ensuring high sample throughput.
720006989, Revised October 2020