This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the fast chiral separation of benzyl mandelate and the enantiomeric excess determination at 0.02% impurity level using the Waters ACQUITY UPC2 System.
The high detection sensitivity of the ACQUITY UPC2 System enables the identification and quantification of enantiomeric impurities in drug substances.
According to the September 2005 issue of Chemical & Engineering News, 9 out of the top 10 drugs (based on sales figures) have chiral active ingredients, and five of those nine drugs have single enantiomeric active ingredients. The single enantiomeric form of a chiral drug is considered an improved chemical entity, which may offer a higher efficacy, a better pharmacological profile, and a more favorable adverse reaction profile. For the manufacturers of single enantiomeric drugs, the undesired stereoisomers should be considered in the same manner as other organic impurities. Regulatory requirements for the identification, quantification, and control of impurities in drug substances and their formulated products have been explicitly defined by the International Conference of Harmonization (ICH). The threshold for identification and quantification of organic impurities is 0.1% for the majority of compounds, according to the ICH.
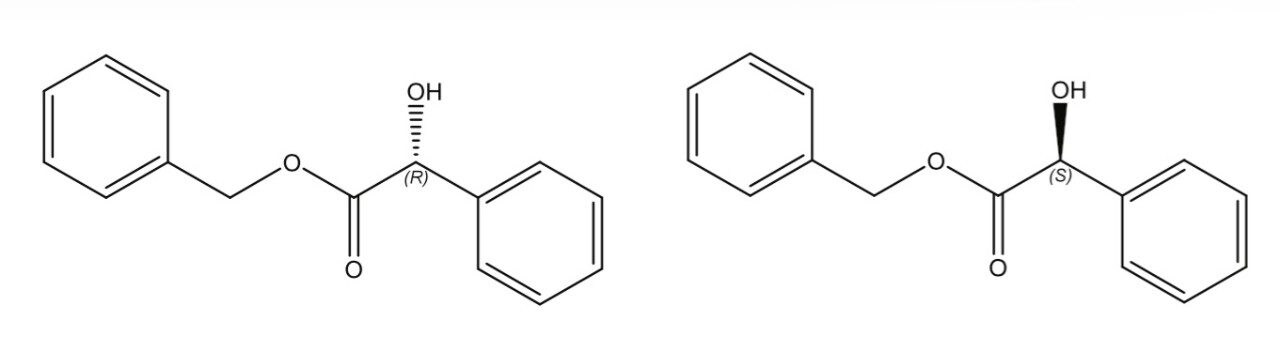
Benzyl mandelate, shown in Figure 1, is an important synthetic intermediate for pharmaceutical synthesis. A racemic mixture of R- and S-benzyl mandelate (0.20 mg/mL in methanol for each enantiomer) was separated using UltraPerformance Convergenc Chromatography (UPC2), and the chromatogram is shown in Figure 2. Key experimental parameters are listed in Table 1.
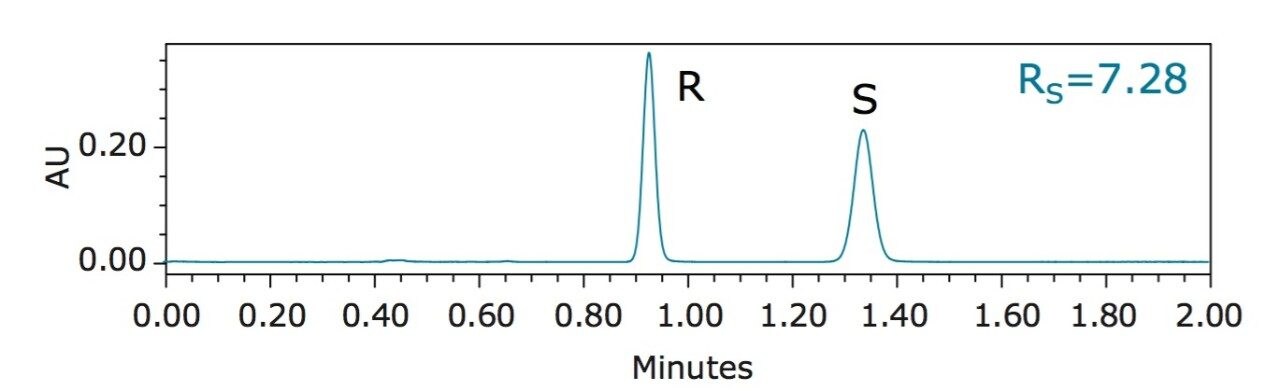

The overall analysis time was less than 1.5 min. Average base peak widths were less than 6 s. Based on the peak area, the ratio of the R- and S-benzyl mandelate was 0.997. Retention time and peak area repeatability measurements were based on five replicate injections, as summarized in Table 2. At 0.20 mg/mL concentration, repeatability for retention time was better than 0.23% RSD and better than 0.5% RSD for peak area.
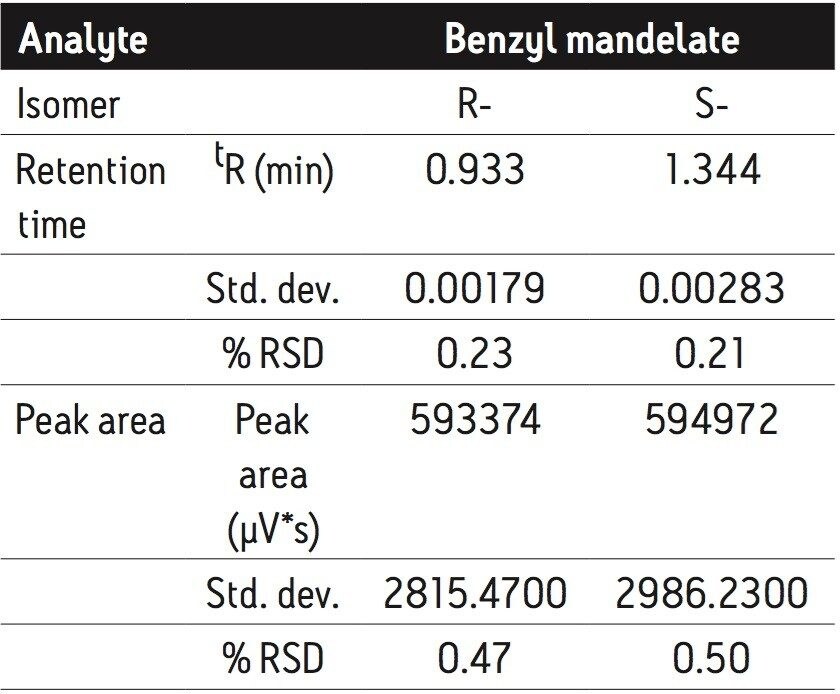
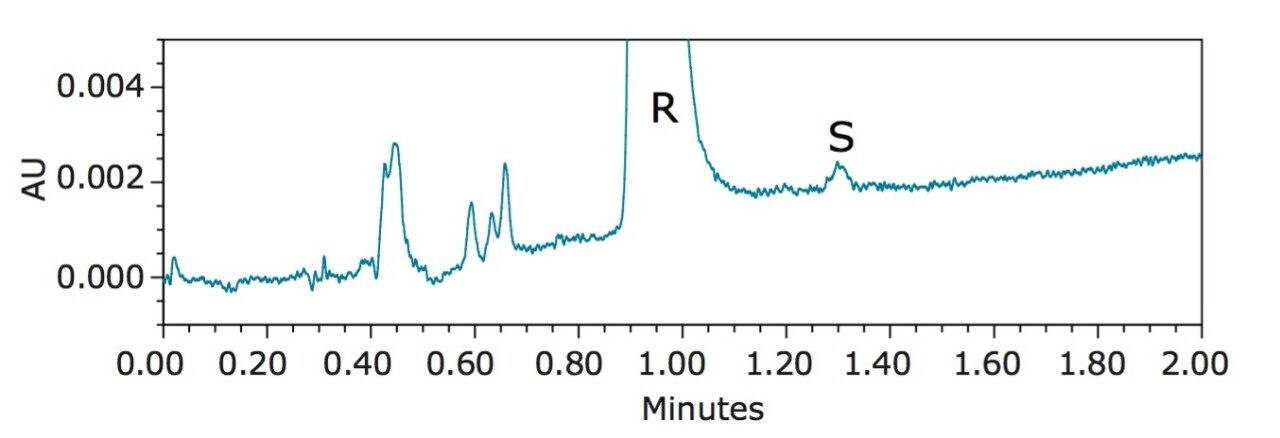
Figure 3 shows the UPC2 chromatogram of R-benzyl mandelate at 2 mg/mL. The minor peak at 1.30 min corresponds to S-benzyl mandelate as confirmed by the UV spectrum (results not shown). This S-benzyl mandelate impurity peak has an S/N of ~3 (LOD) and represents 0.02% of the major peak based on the peak area. This increased detection sensitivity can be attributed to the holistically designed ACQUITY UPC2 System, which includes an improved pumping system and an optimized detector design. The enantiomeric excess (e.e.) in this case was 99.96%
UPC2 chiral separations of R- and S-benzyl mandelate in less than 1.5 min were successfully demonstrated using the ACQUITY UPC2 System. At 0.20 mg/mL concentration of each enantiomer, excellent repeatability (better than 0.23% RSD for retention time and better than 0.5% RSD for peak area) was obtained. Improved detection sensitivity, resulting from a new pumping system and optimized detector design, made detection of a 0.02% enantiomeric impurity and e.e. determination possible. The AQUITY UPC2 System is suitable for the analysis of low level enantiomeric impurities, enantiomeric excess determinations, and QA/QC analyses.
720004245, February 2012