
This study details about detection of nordiazepam and oxazepam in Calliphora Vicina larvae using LC-MS/MS.
In addition to their use in the estimation of postmortem interval, insects may serve as reliable alternate source for toxicological analyses in the absence of tissues and fluids normally taken for such purpose. To date, a variety of compounds have been measured in fly larvae and pupae using different analytical procedures i.e. (Radio-Immunoassay (RIA), Gas Chromatography (GC) and Thin-Layer Chromatography (TLC)). In these studies a minimum of 1g (approximately 20 larvae) was needed to detect the toxic compound.
In this study we used LC-MS/MS (Liquid Chromatography-Tandem Mass Spectrometry) to detect the benzodiazepine Nordiazepam and its metabolite Oxazepam, in single larvae of the Calliphora vicina. Benzodiazepines are prescribed for the symptomatic treatment of anxiety and sleep disorders. They are frequently encountered in postmortem blood analysis (suicide or accidental deaths).
In addition, we compared the development of postfeeding larvae and pupae fed on different concentrations of Nordiazepam.
Flies and larvae were from a stock colony of Calliphora vicina maintained in an environmental chamber at 18–24 °C and 60–70% humidity with cyclical artificial lighting simulating 16 hr daylight and 8 hr darkness.
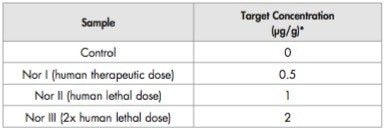
Larvae were reared on artificial food (beef heart) spiked with a range of concentrations of Nordazepam (Table 1). Post-feeding larvae were harvested from day 4 till day 8. Thirty larvae were boiled and conserved (in a mixture of ethanol and acetic acid) prior to measurement of length. Another 30 were used for toxicological analysis. These were weighed and then killed, by freezing to -20 °C. The larvae were stored at -20 °C until analysis.
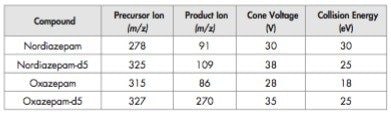
Individual larvae and pupae samples were prepared for LC-MS/MS as follows; the sample was transferred to a vial containing 500 μL water and vortex-mixed thoroughly. One millilitre of acetonitrile (containing deuterated internal standards) was then added and the samples mixed for a further minute. The mixture was evaporated to ~100 μL and then filtered. A 10 μL aliquot was analysed using LC-MS/MS.
|
LC system: |
Alliance 2690 |
|
Column: |
Conventional Phenyl Column (2.5 x 150 mm, 5 μm) |
|
Mobile phase: |
A=10:10:80 acetonitrile:methanol: 20 mM ammonium acetate B=95:5 acetonitrile: 20 mM ammonium acetate |
|
Flow rate: |
0.25 mL/min |
|
Injection volume: |
10 μL |
|
Time (min) |
%A |
%B |
Curve number |
|---|---|---|---|
|
0.0 |
100 |
0 |
1 |
|
0.5 |
75 |
25 |
1 |
|
8.0 |
40 |
60 |
7 (concave) |
|
11.0 |
40 |
60 |
6 (linear) |
|
12.0 |
100 |
0 |
1 |
|
15.0 |
100 |
0 |
1 |
|
Mass spectrometer: |
Quattro Ultima Triple Quadrupole |
|
Ionisation Mode: |
ES+ |
|
Capillary Voltage: |
3 kV |
All larvae, pupae and food spiked with Nordiazepam were positive for the drug, whereas all control samples were negative.
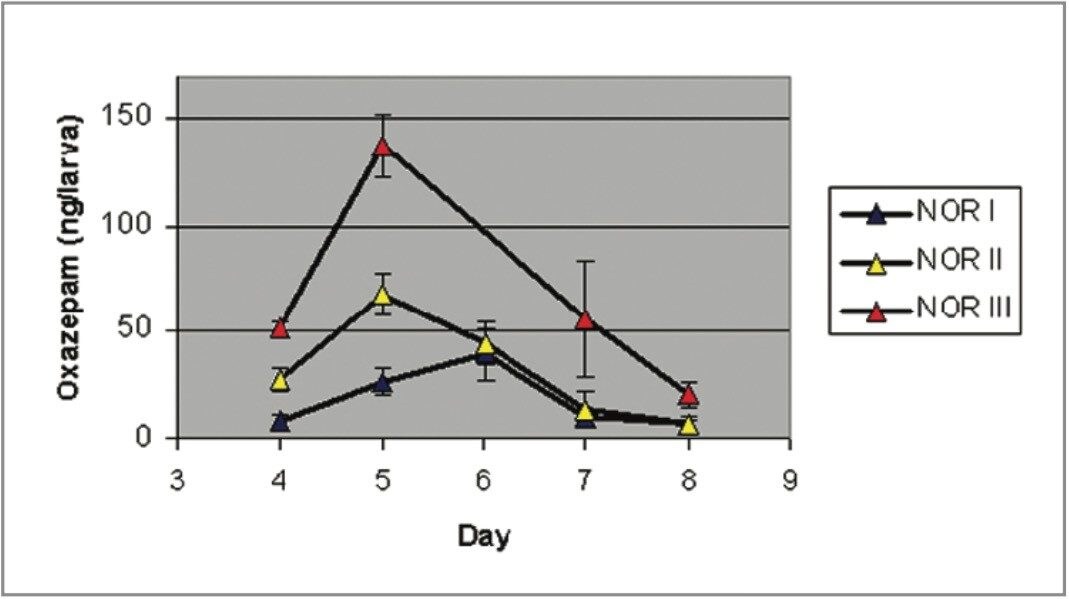
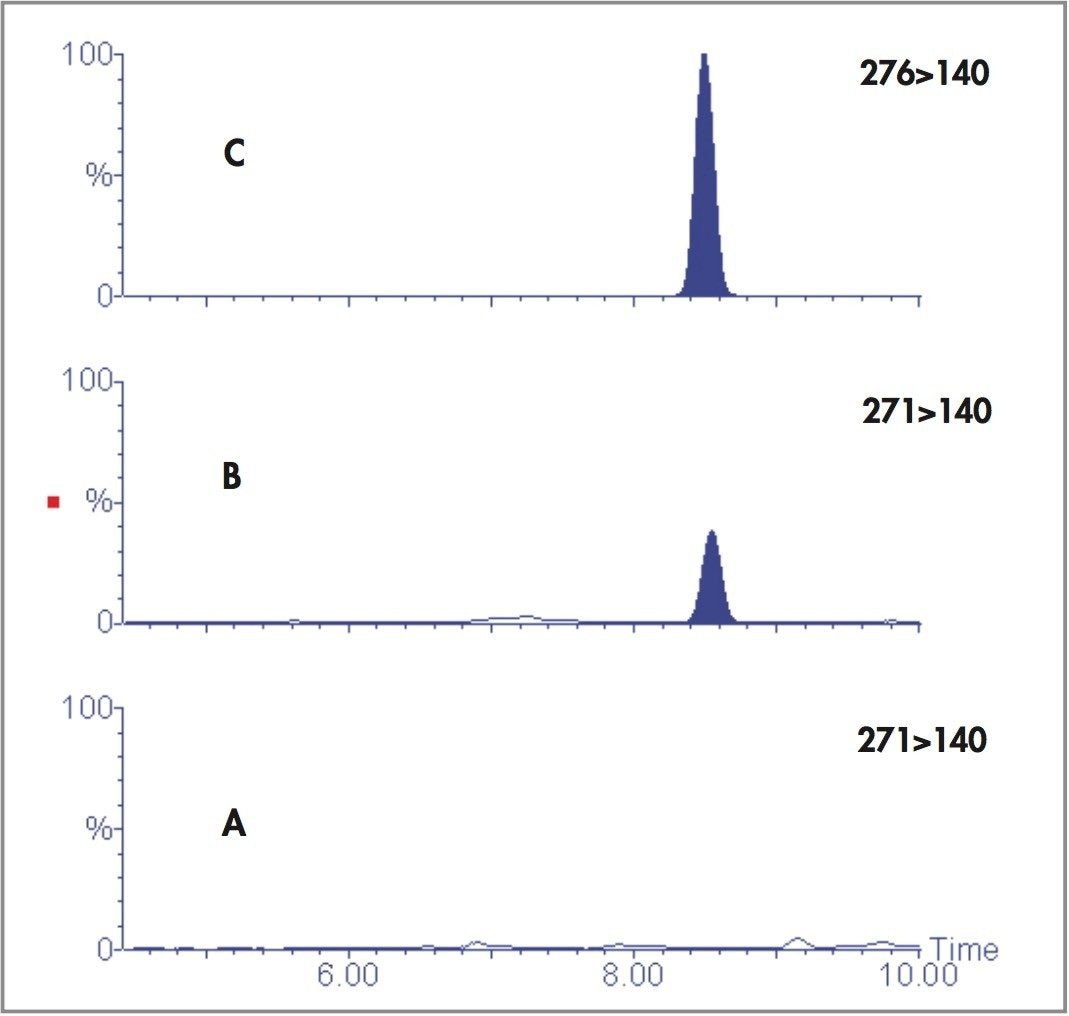
Figures 1 and 2 show the larvae Nordiazepam and Oxazepam concentrations from days 4–8. Figure 3 shows the MRM chromatograms obtained following the LC-MS/MS analysis of a control larva and a Nordiazepam positive larva.
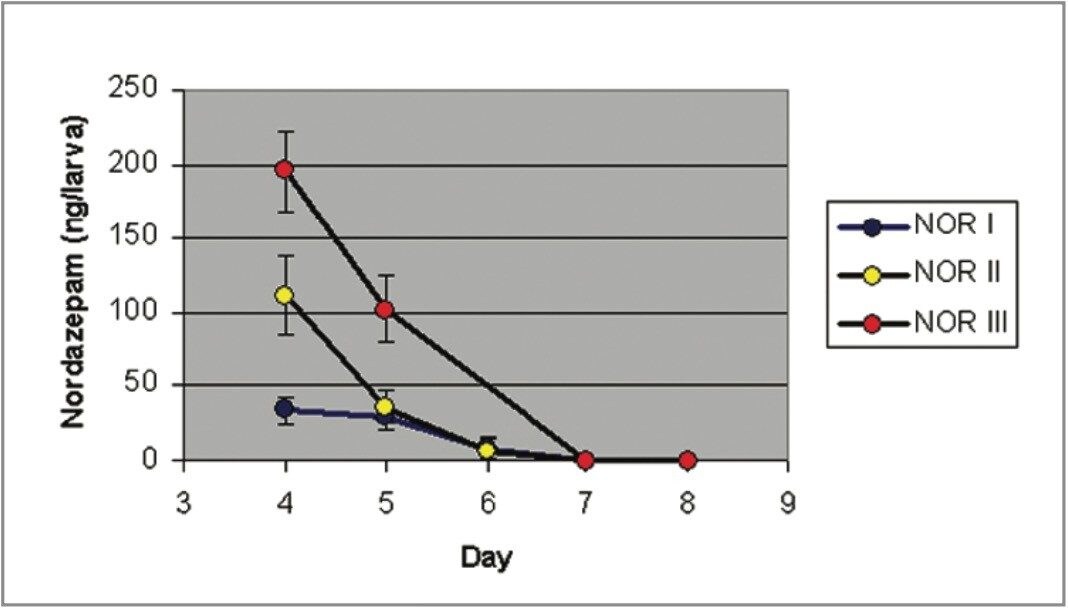
Peak concentrations of Nordiazepam were measured on day 4 for NOR I, II, and III, followed by a precipitous fall of larval Nordiazepam concentrations. From day 7, Nordiazepam was not detectable in a single larva.
Peak concentrations of Oxazepam were measured on day 5 for NOR II and III and at day 6 for NOR I. Low concentrations of Oxazepam were still measured at day 8. In this study, two patterns of development were observed; the post-feeding larvae fed on Control, NOR I, and NOR III food regime developed at approximately the same rate and each demonstrated wandering-phase behaviour at day 6, pupation at day 8, and emerging of adult flies at day 18.
In contrast, the development of larvae fed with the NOR II regime was 1 day later in all stages.
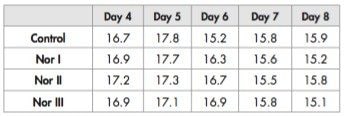
Post-feeding larval length is shown in Table 3; no significant differences were observed.
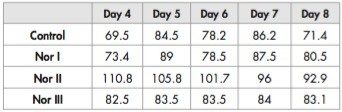
Post-feeding larval weight is shown in Table 4: although no significant differences were seen in larvae reared on Control, NOR I and NOR III food regimes, the mean weight of larvae fed on NOR II was significantly higher. This observation was also confirmed in a second rearing experiment.
We have developed a method that allows the detection of Nordiazepam and its metabolite Oxazepam in single larvae. Larval drug concentrations showed a stepwise increase with increasing drug concentrations in the foodstuff. It was clear that Nordiazepam was metabolized to Oxazepam, which was still detectable at day 8. Nordiazepam was detectable until day 6. Control maggots were negative.
No differences were seen on the post-feeding larval length, but differences in post-feeding larval weight and development were seen in the NOR II larvae. The reason of this disturbance is not yet understood, but is presumably because larval physiology is disturbed to a greater extent by this drug level. This study indicates that an estimation of the postmortem interval based on the length of the post-feeding larvae of Calliphora vicina, which have fed on tissues containing Nordiazepam, will have no error. However an error, of up to 24 hours, can be made if the estimation is based on duration of larvaland puparial stages.
720001550, July 2007