This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates about LCT Premier Mass Spectrometer which eases routine exact mass measurement.
As with every analytical tool, the more accurate your measurement, the more confident you can be with your results. For example, when carrying out a metabolism investigation following an in-vivo experiment, are you sure that a peak detected at m/z 342.1 is an actual metabolite or a matrix-related component, or even a solvent contaminant? Mass measuring the analyte of interest to four decimal places will give you that confidence.
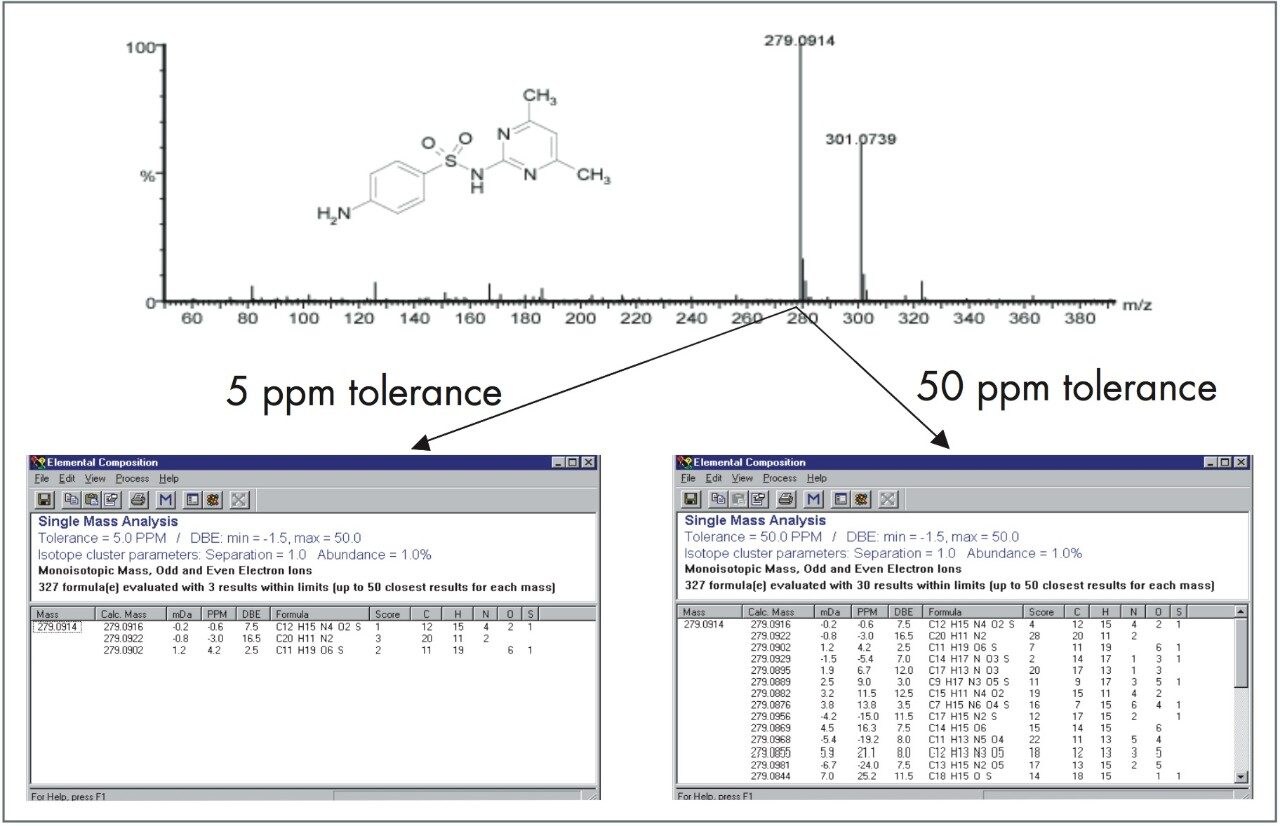
By reducing the error on a mass measurement, the possible elemental formulas that can fit a particular measurement significantly reduce, therefore providing better specificity. Figure 1 clearly explains this improvement gained by exact mass measurement. By measuring to four decimal places with a high degree of accuracy provides a reduced number of potential answers. However, such mass measurement accuracy is not routinely or easily available with typical scanning instruments such as quadrupole or ion trap mass spectrometers. For routine and easy-to-use exact mass measurement in a benchtop instrument, orthogonal acceleration time of flight (oa-Tof) mass spectrometry provides the solution.
Orthogonal acceleration time of flight and exact mass measurement can allow you to:
With the Waters LCT Premier oa-Tof mass spectrometer, you can acquire high resolution LC-MS data that allows exact mass measurements to be easily generated, to within 3 ppm of an analyte’s actual mass. In addition to the next generation of instrument electronics and oa-Tof optics, the LCT Premier is equipped with a LockSpray ion source as a standard.
LockSpray is our novel dual electrospray ion source that was designed to make exact mass measurement easy (Figure 2). Your LC column is connected to the standard ESI sprayer while the single point “lock mass” used to exact-mass measure your data is introduced into the separate reference sprayer. The eluent from each of the two sprayers is kept completely separate by a baffle that is indexed via software control for total data integrity. The reference sprayer is sampled periodically during an LC-MS experiment and the data generated is used automatically to exact-mass measure the analyte data.
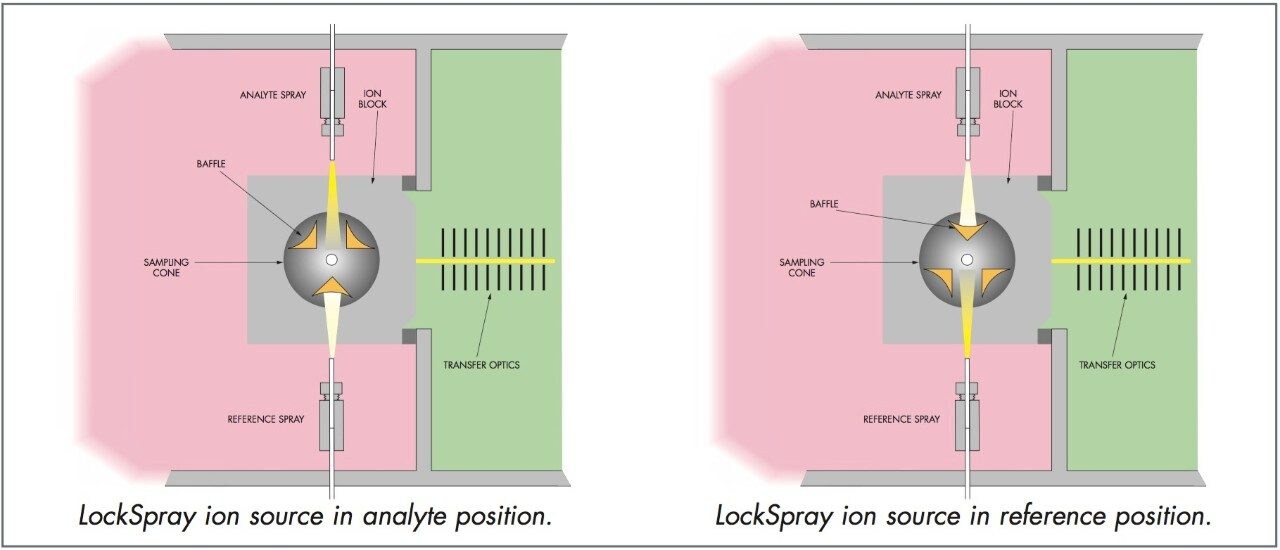
With LockSpray, detrimental issues associated with traditional exact mass experiments (i.e. teeing in a reference mass post-column, etc.) are overcome. Experimental problems include plumbing issues introducing the “lock mass”, potential mass interferences between analytes of interest and the “lock mass” and changes in the “lock mass” response with changing LC gradients. Figure 3 highlights the effectiveness of LockSpray, even in the presence of high intensity chromatographic peaks over long run times. The upper trace shows the reference sprayer response and how it remains constant, ensuring that there is always a reference mass present to guarantee the exact mass measurement.
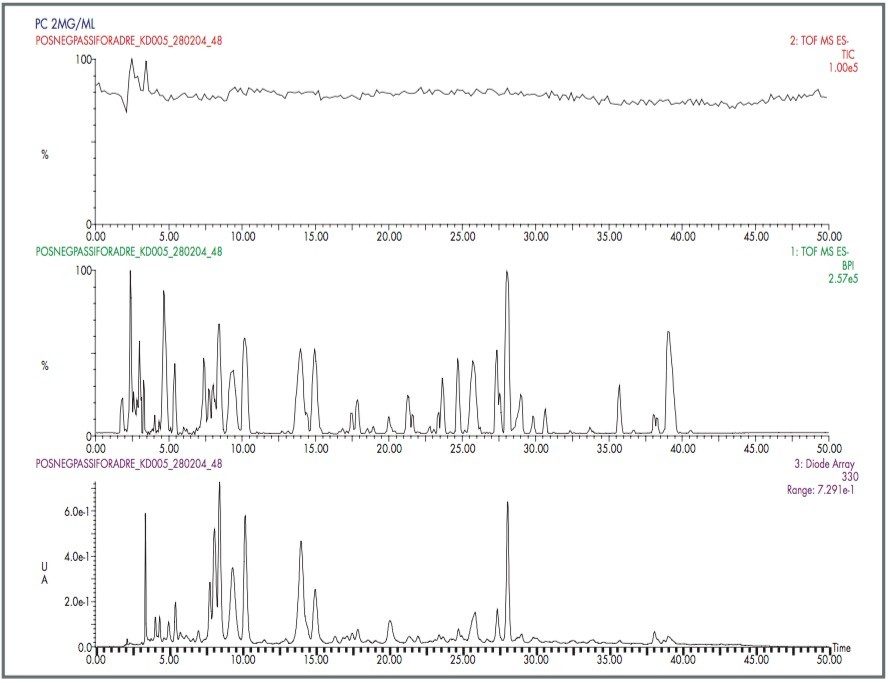
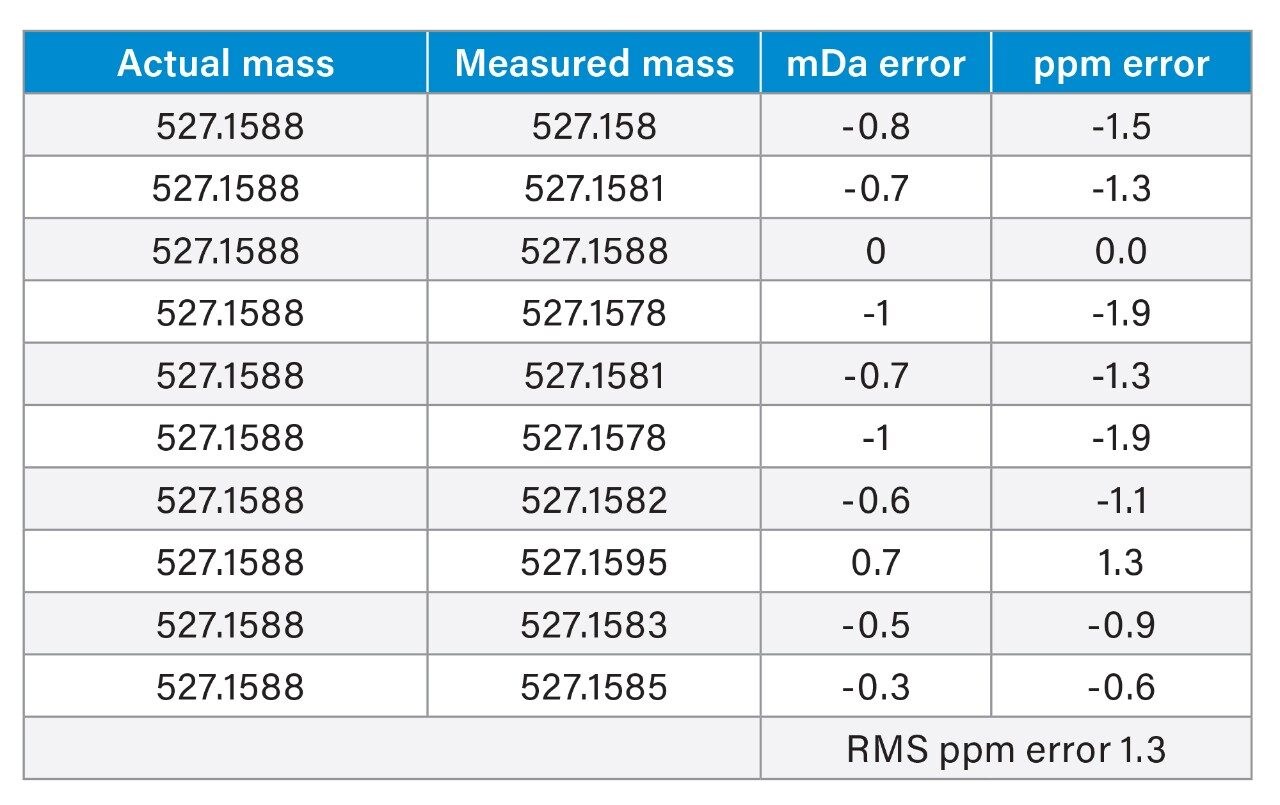
Table 1 below lists the exact mass measurements along with the calculated mDa and ppm errors from the theoretical for the exact mass measurement of raffinose [M+Na]+. LockSpray and a single “lockmass” was used. The RMS ppm error for all 10 measurements was calculated at 1.3 ppm.
LockSpray and the LCT Premier Mass Spectrometer have been used to analyse an eight-component mixture of “pharmaceutical-like” compounds. Each compound was analysed by LC-MS and an exact mass was generated for each peak. Figure 4 shows the typical LC-MS chromatogram obtained, and Table 2 shows the exact mass measurements for each of the peaks. The RMS ppm error for all the exact mass measurements was 1.6 ppm.
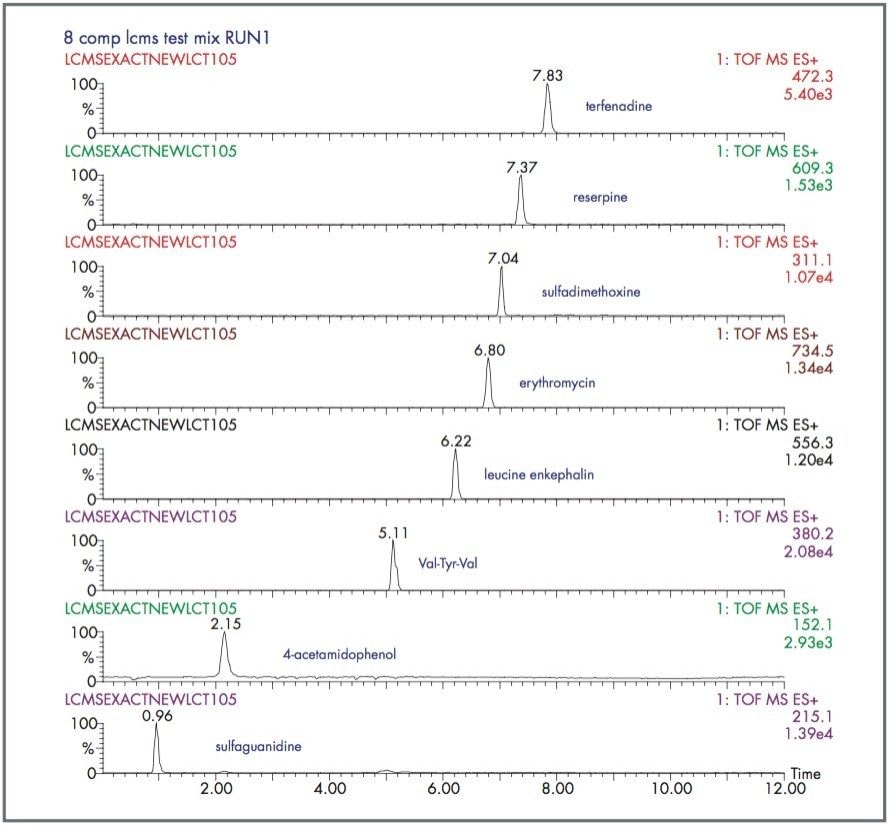
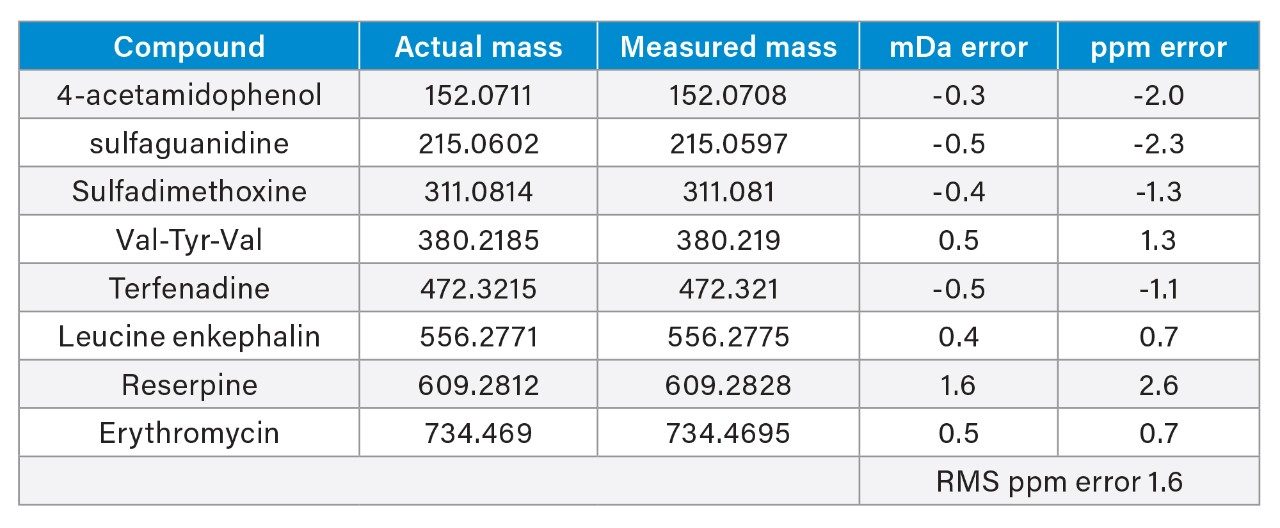
This example shows that the LCT Premier provides excellent mass measurement accuracy across a wide range of compounds with varying mass, physical properties and retention time during a reversed phase LC gradient. This provides improved specificity during an LC-MS experiment, providing the analyst with increased confidence in the analytical result.
The LCT Premier Mass Spectrometer also has the capability of carrying out positive/negative ionisation per sample and maintaining exact mass measurement. All this is done with an interscan delay of 300 milliseconds. This technique is ideal for compound screening applications where the particular mode of ionisation is not known. Figure 5 shows an exact mass measured spectrum of raffinose in both positive ([M+Na]+) and negative ([M-H]-) ionization with an interscan delay of only 300 milliseconds.
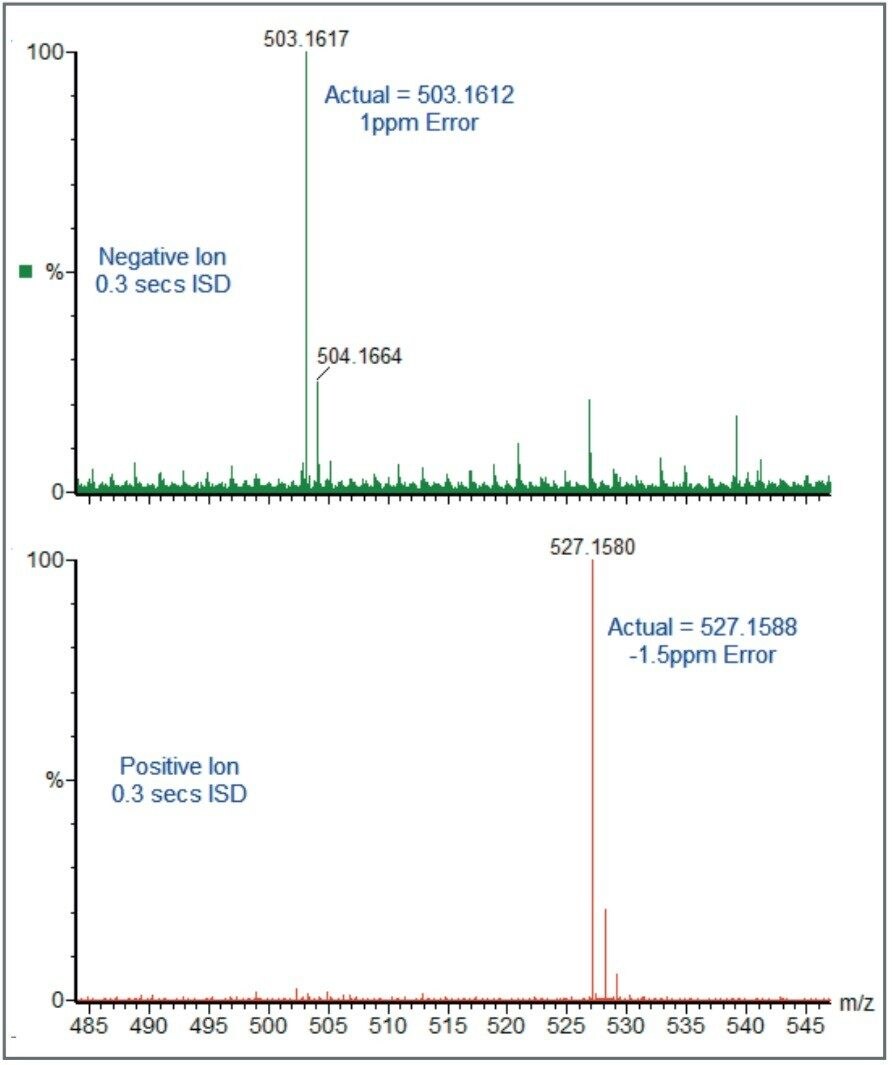
Exact mass measurement is an invaluable technique for analyzing either known or unknown analytes. With the possibility of performing an elemental composition calculation from the exact mass measurement, peaks of interest can be assigned as “real” or matrix related. Exact mass provides the analyst with an extra level of confidence in the analytical answer.
One application where exact mass measurement is very useful is for metabolite identification studies. LC-MS analysis of metabolism studies often result in complex chromatograms containing many peaks. With an exact mass measurement, the target analytes can be confirmed as being present; in addition, any unexpected metabolites can be distinguished from the background with a high degree of confidence.
Exact mass measurement can also be applied to fragment ions to provide another level of confirmation. Although the LCT Premier does not have dedicated MS/MS capability, in-source fragmentation can be performed.
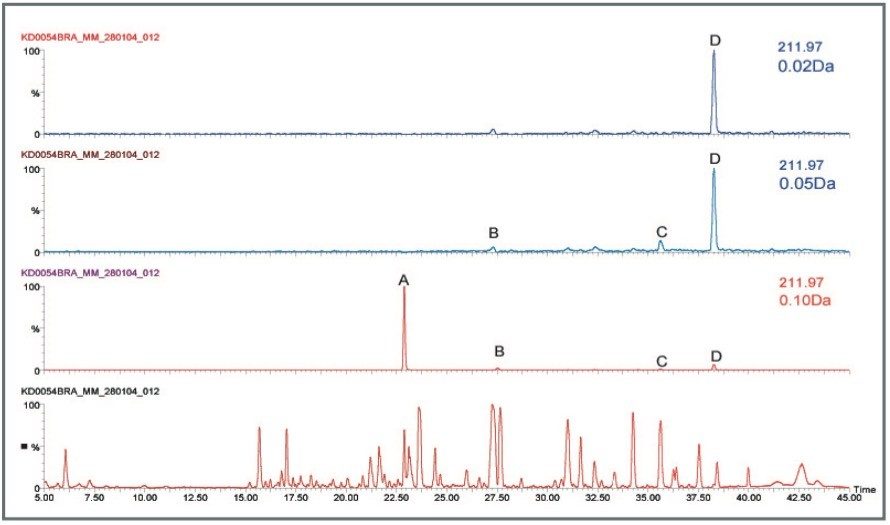
Exact mass measurement also provides specificity when determining unknown samples or dealing with complex mixtures. Figure 6 shows the benefits of using an “exact mass window” around the peak of interest. By using a 0.1 Da window around the mass of interest, peak A may be considered as the right peak. However, as the window around the mass of interest is made smaller and thus making the measurement more accurate, peak D one of the smaller peaks clearly becomes the correct peak of interest. Exact mass measurement becomes invaluable when dealing with complex sample analysis.
The LCT Premier Mass Spectrometer: routine exact mass measurement made easy.
720000930, September 2004