This is an Application Brief and does not contain a detailed Experimental section.
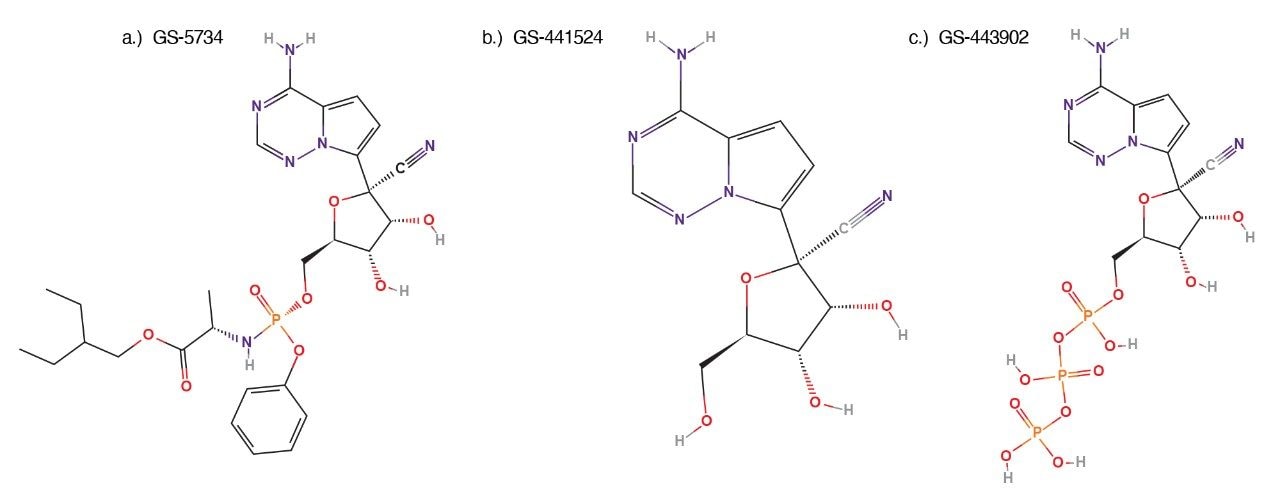
Nucleic acid polymerases are enzymes that catalyze the replication and transcription of genetic information, so it is not surprising that they are also a very important druggable target. Structural differences between viral polymerases and human polymerases has given hope for efficacious antiviral treatments. Viral DNA or RNA polymerases can thus be targeted with substrate mimicking inhibitors. In the case of SARS-CoV-2, an RNA polymerase is responsible for genome replication. Numerous antivirals with promising in vitro efficacy against the novel coronavirus are being tested on patients. One of these has even been approved by the FDA as a treatment for COVID-19. This molecule is remdesivir (GS-5734), a phosphoramidate prodrug that is converted intracellularly into an active triphosphate form. The promise of these antivirals and others like them has led to an explosion of new drug discovery projects and clinical studies.
In some cases, it is of interest to monitor biotransformation events by means of liquid chromatography paired with optical detection or with mass spectrometry. To date, nucleotide analogs have largely been separated using ion pairing reagents, like tributylamine.1 This serves to improve the retention of the hydrophilic, acidic analytes, and it suppresses problematic metal adsorption-based sample losses. At the same, a strong ion pairing agent, like tributylamine, can suppress ionization and make it a challenge to switch back to other LC-MS techniques.
In this application note, we take advantage of hybrid silica surfaces and mixed mode chromatography with ammonium acetate mobile phase to devise a starting point for a method that could potentially be applied to in vitro cell assays or pharmacokinetic analyses and dosing studies.
For more information about this application or to learn how Waters can help you in your efforts against COVID-19, please contact COVID19Response@waters.com
Remdesivir (GS-5734, Fig. 1a) is an investigational small-molecule antiviral drug that has demonstrated activity against RNA viruses in several virus families, including coronaviruses. Remdesivir is a prodrug of a nucleoside, GS-441524, (Fig. 1b), both of which are metabolized intracellularly into the active nucleoside triphosphate (GS-443902, Fig. 1c).5 Originally developed to treat Ebola virus infection,2 remdesivir has been the focus of extensive research focusing on repurposing antiviral medications to be used alone or in combination with other therapeutics for the treatment of the SARS-CoV-2 infection.4
One challenge that was anticipated in the separation of these molecules was the retention and peak shape of the nucleoside triphosphate. While separations have been achieved using ion-pairing reagents and HILIC mode chromatography, we chose to investigate the use of mixed-mode chromatography with ammonium acetate buffers to achieve a simple, fast analysis that could be used with either optical or MS detectors.
Mixed-mode chromatography (MMC) achieves analyte separation by utilizing multiple types of interactions between the stationary-phase and the analyte. Mobile phase pH, ionic strength and organic content are all factors that can influence the retention and selectivity of analytes. The Atlantis BEH C18 AX stationary-phase is a mixed-mode reversed-phase/anion-exchange stationary phase based on bridged-ethyl hybrid particles.3 The utilization of bridged-ethyl hybrid particles allows for the use of a wide range of mobile phase pH values and the presence of both C18 groups and anion-exchange groups provided the ability to separate analytes based on either their hydrophobic or ionic characteristics. Additionally, Atlantis BEH C18 AX stationary-phase is the first chromatographic material that has been packed using MaxPeak High Performance Surfaces Column hardware, designed to reduce acidic analyte interactions with stainless steel.6
The following experimental conditions were used to analyze remdesivir (GS-5734), its parent nucleoside (GS-441524), and the nucleoside triphosphate (GS-443902) using an Atlantis Premier BEH C18 AX Column.
|
LC system: |
ACQUITY Premier Quaternary System |
|
Detector 1: |
ACQUITY Premier PDA Detector |
|
Detector 2: |
ACQUITY QDa Mass Detector |
|
Column: |
Atlantis Premier BEH C18 AX 1.7 μm Column (2.1 x 50 mm) |
|
Column temp.: |
50 °C |
|
Sample temp.: |
12 °C |
|
Injection volume: |
1 μL |
|
Flow rate: |
0.5 mL/min |
|
Mobile phase A: |
100% Acetonitrile |
|
Mobile phase B: |
IonHance CX-MS Concentrate A, pH 5 (1:5 Dilution) p/n: 186009280 |
|
Mobile phase C: |
100% 18Ω water |
|
Mobile phase D: |
IonHance Ammonium Acetate pH 6.8 Concentrate (1:5 Dilution) p/n: 186009705 |
Standards were purchased from multiple sources. Remdesivir (GS-5734, Fig. 1a) was purchased from Ambeed (Arlington Hts, IL 60004, USA), remdesivir nucleoside (GS-441524, Fig. 1b) was purchased from Biosynth-Carbosynth (Itasca, IL 60143, USA), and the remdesivir nucleoside triphosphate (GS-443902, Fig. 1c) was purchased from AOBIOUS, Inc (Gloucester, MA 01930, USA).
Two standards containing both remdesivir and the remdesivir nucleoside triphosphate were prepared in the concentration ratios of 500: 50 μg/mL and 50: 500 μg/mL. These ratios were prepared to mimic two different timepoints from dosing and the start of metabolic conversion. A single component standard of remdesivir nucleoside was prepared at 10 μg/mL and 100 μg/mL concentrations.
Chromatographic mobile phases were prepared on-line using a quaternary pump with IonHance buffer concentrates (which contain 20% (v/v) acetonitrile). The buffer concentrates were prepared with 1:5 dilution to achieve final concentrations of 100 mM in 4% acetonitrile for the IonHance CX-MS Concentrate A, pH 5 and 200 mM in 4% acetonitrile for the IonHance Ammonium Acetate pH 6.8 Concentrate. The 1:5 dilutions were mixed with 18 MΩ water and acetonitrile to form the gradient. The final gradient was 5 mM ammonium acetate pH 6.8 in 0% acetonitrile to 20 mM ammonium acetate pH 6.8 in 60% acetonitrile in 4 minutes using a linear gradient (curve 6) and return to initial in 0.5 minutes. A longer 8-minute gradient was also run with good results (Figure 2).
Remdesivir has a moderate logP value of 2.01, so it was predicted that a relatively high percentage of acetonitrile would be required to elute the prodrug. It was also predicted that pH would have a critical effect on the retention of the nucleoside triphosphate.
The retention factor for remdesivir was calculated for a series of injections made using isocratic elution conditions and mobile phases prepared with an acetonitrile content range of 40 to 60% in 10 mM ammonium acetate. Additionally, the effect of pH values 4.8 and 6.8 was evaluated.
To achieve retention factors that were greater than one but less than 10, remdesivir required that at least 40 to 60% acetonitrile be used, regardless of the pH of the aqueous mobile phase. A higher organic endpoint was used preferred for the potential application of the method to additional, more hydrophobic analytes. Like in the screening of conditions for remdesivir, two pH values were also used to analyze remdesivir nucleoside triphosphate. However, most of the analyses were conducted using the preferred pH of 6.8. The higher pH produced sharper peaks than the pH 4.8 mobile phase, albeit with slightly lower resolution between the diphosphate and triphosphate forms. If desired, mobile phase pH can thus be adjusted to fine tune the separation.
A comparison of the two standards is shown in Figure 2. The top chromatogram (Fig. 2a) was obtained from a sample comprised of remdesivir at 10 times the concentration of remdesivir nucleoside triphosphate, while the bottom chromatogram (Fig 2b) was obtained from a sample containing the opposite ratio: 1-part remdesivir to 10 parts remdesivir nucleoside triphosphate. The mass load-on-column for remdesivir was approximately 0.8 nmol for Figure 2a and 0.08 nmol for Figure 2b, which is comparable to the experimental conditions previously used by Gilead Sciences for bioanalysis studies.1
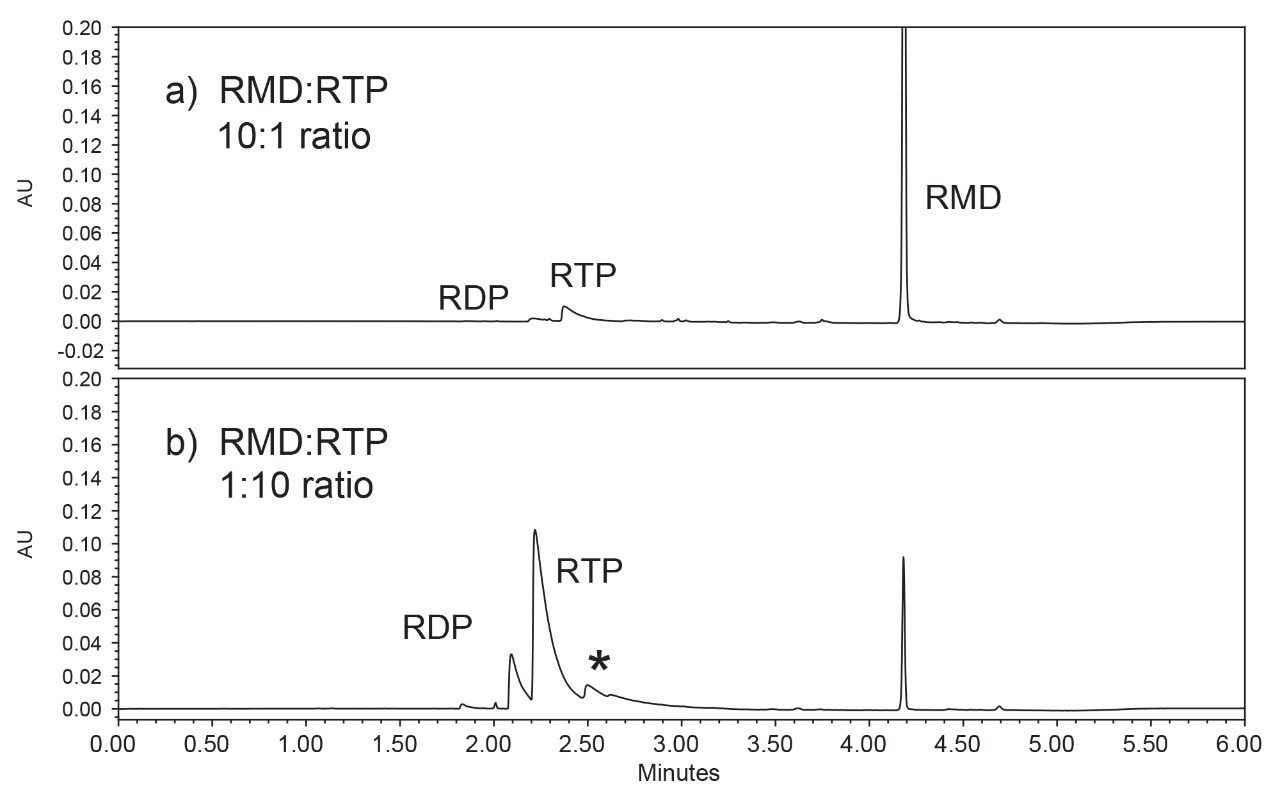
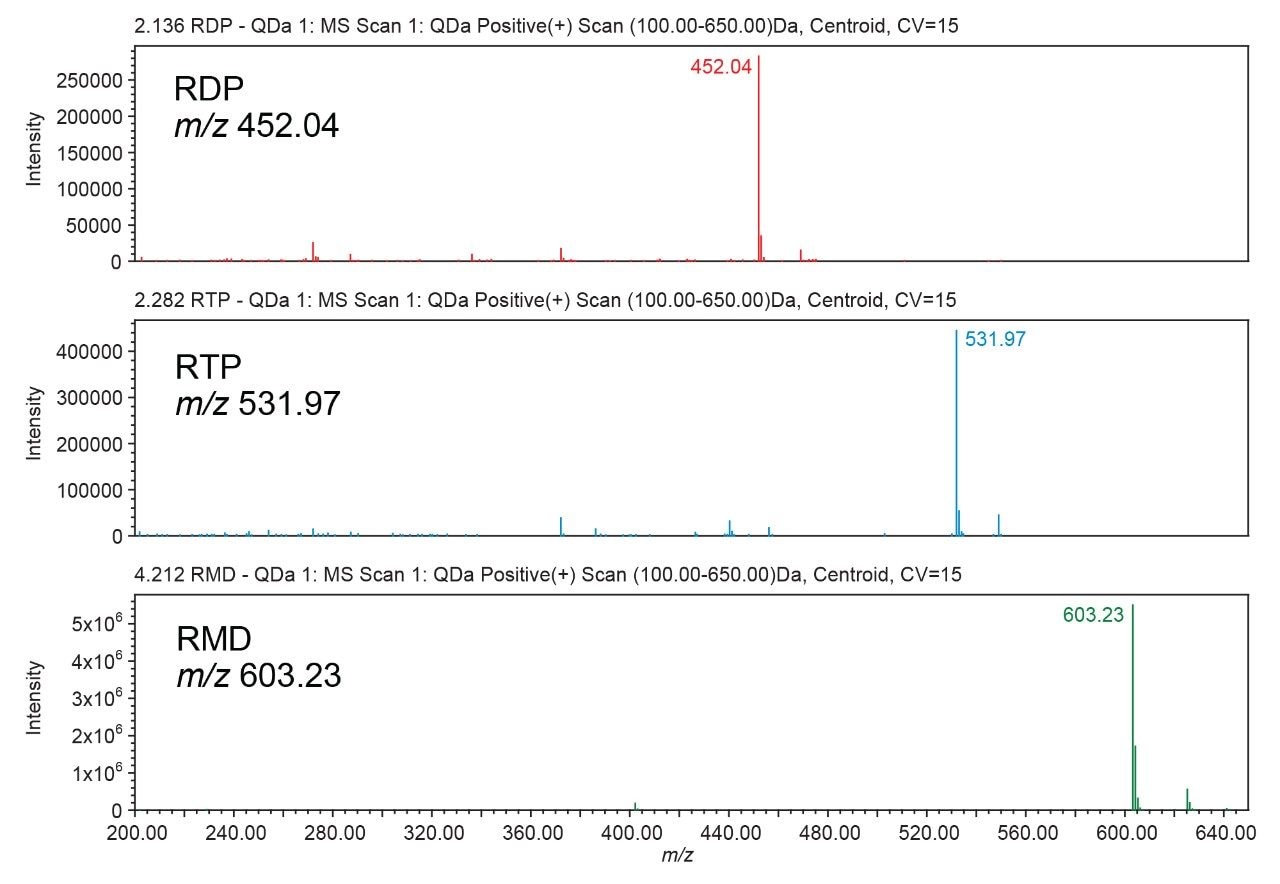
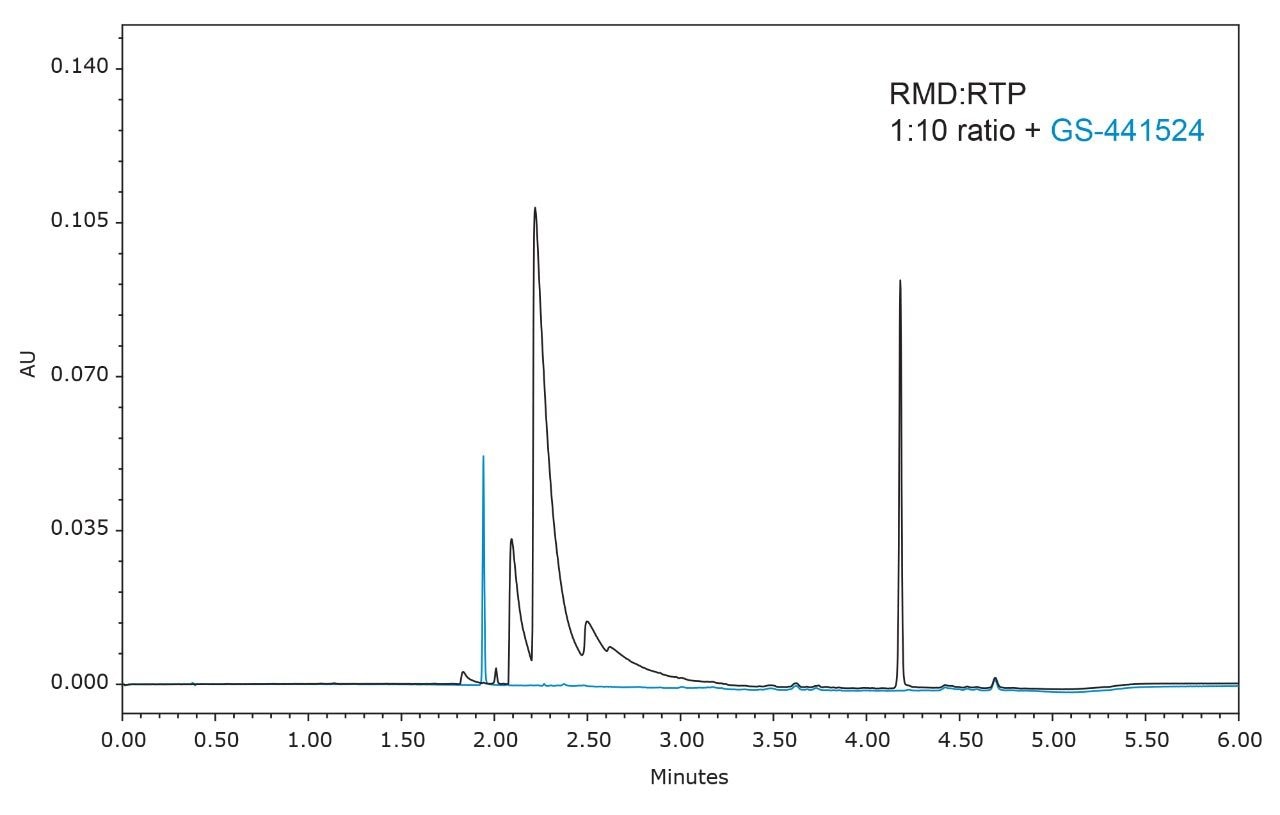
Peak identification was confirmed using an ACQUITY QDa Mass Detector and extracting the m/z values for each analyte (Figure 3). The final gradient was also used to analyze the remdesivir nucleoside (GS-441524) (Figure 4). While this analyte elutes earlier in the gradient and does not interfere with the nucleoside triphosphate, it may provide a new starting point for additional gradient optimization.
A mixed-mode stationary phase, known as Atlantis BEH C18 AX, offers unique selectivity and was therefore considered as a starting point for devising a method to analyze samples containing analytes with multiple functional groups.
In this application note, we took advantage of the pH stable hybrid silica surfaces and operated the mixed-mode column at pH 6.8, a pH which may lead to degradation if used with traditional silica-based reversed-phase/mixed-mode stationary phases. The Atlantis BEH C18 AX mixed-mode stationary-phase has both C18 and anion-exchange groups and each can contribute to a chromatographic separation. At pH 6.8, the anion-exchange sites were tuned to elute remdesivir triphosphate within the gradient, while the C18 groups played the greater role in the retention of remdesivir. The primary goal of good retention for remdesivir triphosphate and resolution from remdesivir was achieved using these conditions. Additional method optimization could be undertaken to improve the peak symmetry for remdesivir triphosphate, such as reducing mass injected on-column.
Ammonium acetate mobile phases prepared without the use of ion-pair reagents were used in the separation, allowing the option of using optical as well as MS detection. Fast and easy mobile phase preparation was accomplished by using readily available, MS-certified buffer concentrates.
With the emergence of new drug discovery projects, analysts might look to apply this method to in vitro cell studies. Likewise, with further optimization, this technique might prove to be attractive for the analysis of polymerase inhibitors and their metabolites as prepared from biological fluids.
720007146, February 2021