Parallel Column Regeneration for Increased Analytical Throughput of Serum Steroid Hormones in Clinical Research
This is an Application Brief and does not contain a detailed Experimental section.
Abstract
Benefits
- 18% increase in throughput of steroid hormone analysis compared with single channel separations
- Efficient shared calibration approach demonstrates equivalence with single channel analysis
Introduction
Extended gradient chromatographic separations are sometimes needed to resolve matrix and isobaric interferences when analyzing complex mixtures by liquid chromatography, Tandem Mass Spectrometry (LC-MS/MS). This can create a challenge for clinical research laboratories striving to meet throughput demands.
Throughput can be limited when routine, single column gradient LC analyses use one pump to load and separate one sample at a time, with data acquisition running in a series that cannot progress until the column has completed a wash and re-equilibration (regeneration) cycle. This approach can be made more efficient by taking the active column offline and using a second LC pump for regeneration. A second column can then be brought online for loading, separation and analysis of the next sample, while the first column is regenerating offline. This approach is termed ‘parallel column regeneration.
Here, we demonstrate the time-savings achieved when parallel column regeneration is applied with the rapid clinical research method for the quantification of androstenedione (A4), testosterone (T), 17 hydroxyprogesterone (17OHP), dihydrotestosterone (DHT), dehydroepiandrosterone (DHEA), and progesterone (P) in human serum using LC-MS/MS. The validity of applying a single calibration curve to results generated on both columns is explored.
The test system, an ACQUITY™ UPLC™ I-Class PLUS SM-FL BSM/BSM Parallel Column Regeneration System with Single Elution Pumps (p/n: 176005409), is shown in Figure 1. Analytes were detected and quantified in the eluted samples using a Xevo™ TQ-S Micro Tandem Mass Spectrometer, operating in positive electrospray ionization mode, and with multiple reaction monitoring (MRM) acquisitions.

Experimental
Sample Preparation
Calibrators, proficiency testing samples from the UK NEQAS proficiency testing scheme, and quality control samples were prepared using stable 13C isotope labelled internal standards, and mixed mode solid phase extraction, as described in Waters™ application note 720006320, with the modification that MassTrak™ Steroid Serum Cal Set 1 (p/n: 186009311IVD) and QC Set 1 (p/n: 186009312IVD) were used.
Data were collected for both parallel and single column regeneration, for comparison purposes.
LC Conditions
|
LC system: |
ACQUITY UPLC I-Class PLUS SM-FL Parallel Column Regeneration System with Single Elution Pumps |
|
|
Sample needle/loop: |
20 µL / 50 µL |
|
|
Sample syringe: |
250 µL |
|
|
Column: |
CORTECS UPLC C18 1.6 µm, 2.1 x 50 mm |
|
|
Precolumn: |
0.2 µm pore size inline filter |
|
|
Mobile phase A: |
0.05 mM Ammonium fluoride (aq) |
|
|
Mobile phase B: |
Methanol |
|
|
Weak needle wash: |
45% (v/v) Methanol (aq) |
|
|
Strong needle wash: |
1/1/1/1 Methanol/Acetonitrile/Isopropanol/Water |
|
|
Column temperature: |
50 °C |
|
|
Injection mode: |
Partial Loop |
|
|
Sample temperature: |
8 °C |
|
|
Load ahead: |
Disabled |
|
|
Active preheater: |
Enabled |
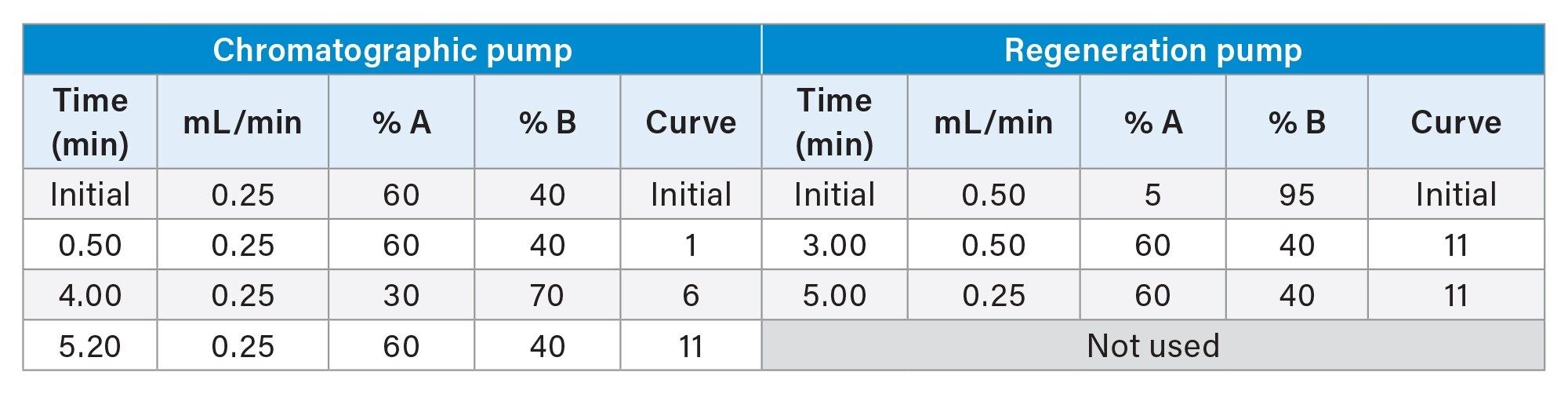
Parallel column regeneration inlet methods were adapted from the single column LC method described in application note 720006320, as summarized in Table 1.
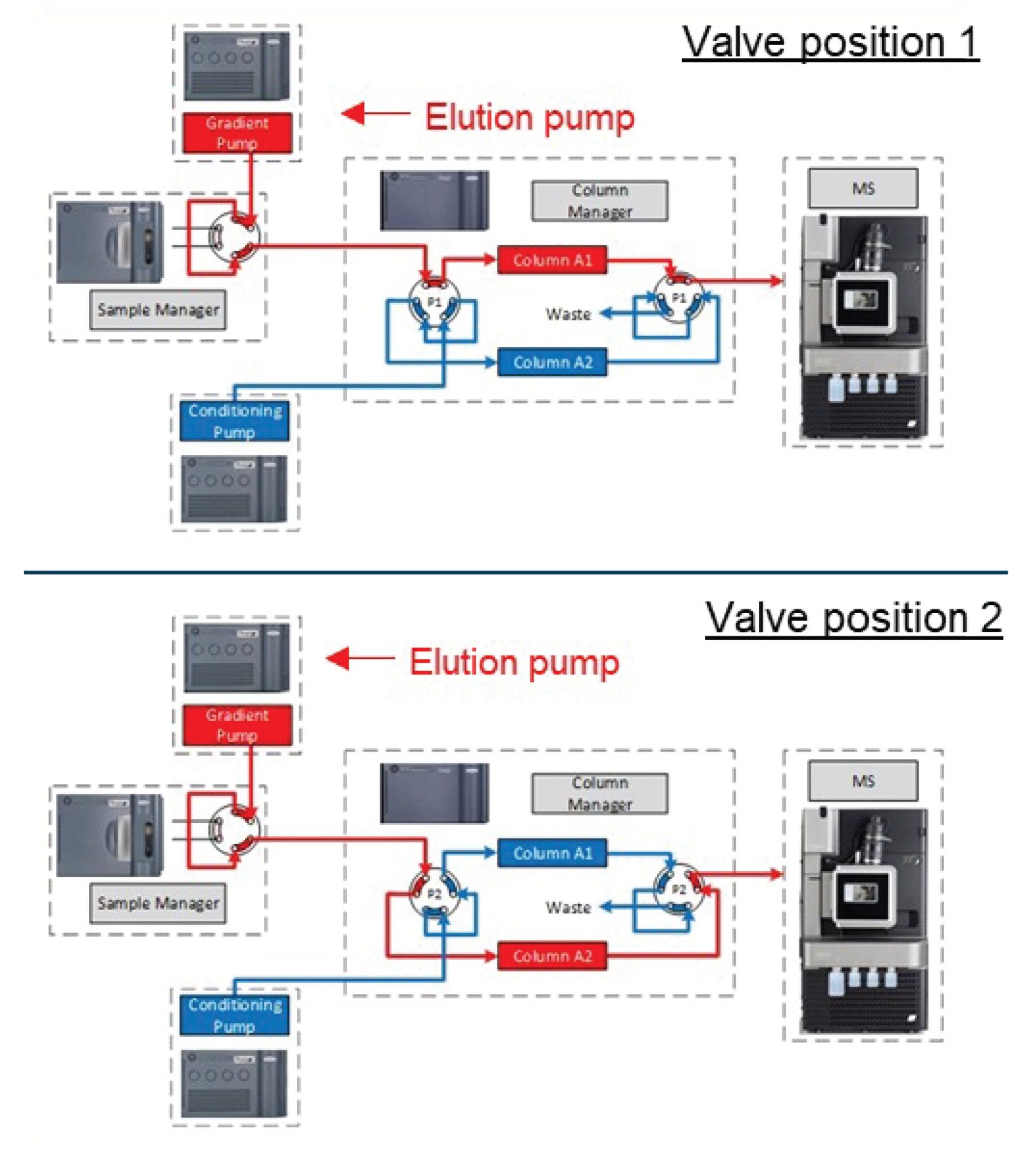
Two LC columns from one stationary phase particle batch were tested. The inlet modules were connected as shown in Figure 2.
The Binary Solvent Managers were configured using MassLynx™ v4.2 instrument control software. The gradient profile for each pump is shown in Table 1. The Run Time was 5.30 minutes.
MS Conditions
The quantifier and qualifier MRM ion transitions and the suggested MS parameters can be found in application note 720006320.
Calibration and Quantification
MassTrak Endocrine Steroid Calibrators were analyzed in entirety, in sequence for single column regeneration, and, by splitting the series between the two columns for parallel column regeneration (i.e. Cal 0 on column 1 and column 2, Cal 1 on column 1, Cal 2 on column 2, Cal 3 on column 1, and so on). Using this design for the five-day, quintuplicate precision studies, three control results were acquired for the first column position, and two control results for the second column position, within each run. The starting column manager position was alternated between batches to ensure an equal division of control data points for the verification studies.
Statistical Analysis
Combined standard measurement uncertainty was estimated for both regeneration techniques using external quality assurance (EQA for the bias component; n=15) and MassTrak Endocrine Steroid Control samples (tri-level, for the precision component; refer to certificate of analysis for p/n: 186009313IVD for target concentrations, n=40). The 40 replicates included the five-day quintuplicate imprecision study data, and an extra run of 15 samples for within-batch repeatability assessment. Expanded measurement uncertainty had a coverage of two standard deviations (encompassing the mean and 95% confidence interval). Agreement between quality control sample results was tested by comparing parallel column results with the single column 95% confidence intervals.
Agreement over a wider range of concentrations was tested using EQA samples. Zeta (z) scores were calculated for the pairs of results returned using both techniques. The standard uncertainty derived from precision studies was used in the calculation of EQA z-scores. EQA samples were arbitrarily categorized as low, medium and high concentration, and the z-score was calculated as the quantitative difference between the techniques, normalized for the square root of the sum of squared uncertainties appropriate for the low, medium or high concentration EQA sample (from either low, medium or high concentration control sample analysis). Good agreement of results was indicated by z-scores between -2 and 2.
Results and Discussion
The correlation coefficient of the linear regression of the calibrators through method verification was ≥0.999 and ≥0.997 (3 s.f.) for single, and parallel column regeneration, respectively (n=5 analyses). Refer to certificates of analysis for p/n: 186009311IVD for details of ranges covered by the MassTrak Endocrine Calibrator set.
Separating the detection and regeneration phases across two channels reduced the injection cycle time from 7.2 to 6.1 minutes per sample, which equates to an analysis time saving of 1.8 hours for a full 96-well plate of prepared samples.
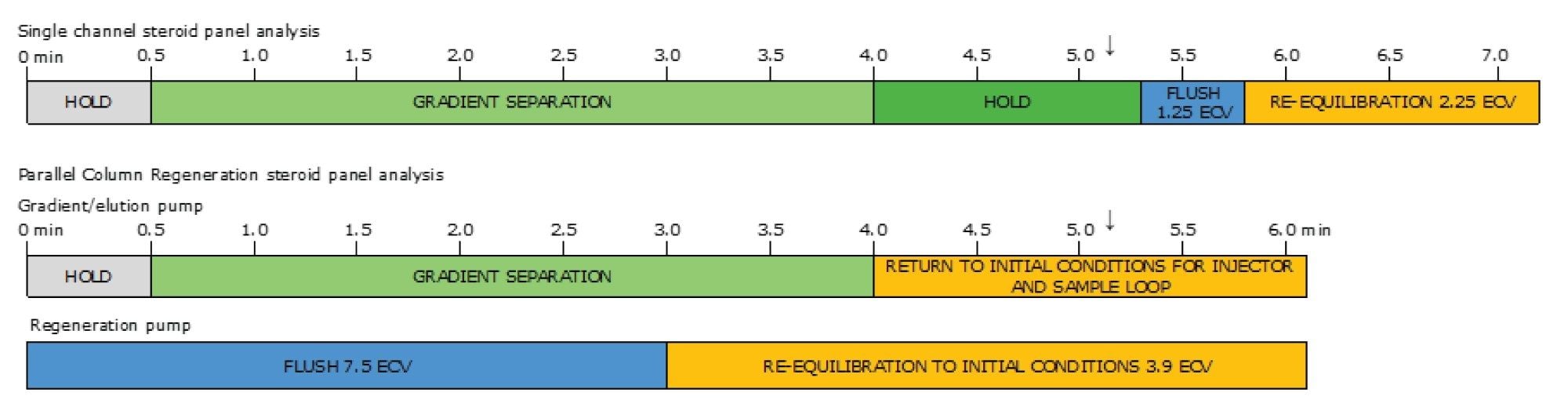
Taking regeneration offline also allowed increased column flushing and equilibration (Figure 3), potentially removing more residual sample matrix from the LC column, and giving the opportunity to create optimal conditions for robust and stable chromatographic performance of early eluting, relatively less-retained analytes.
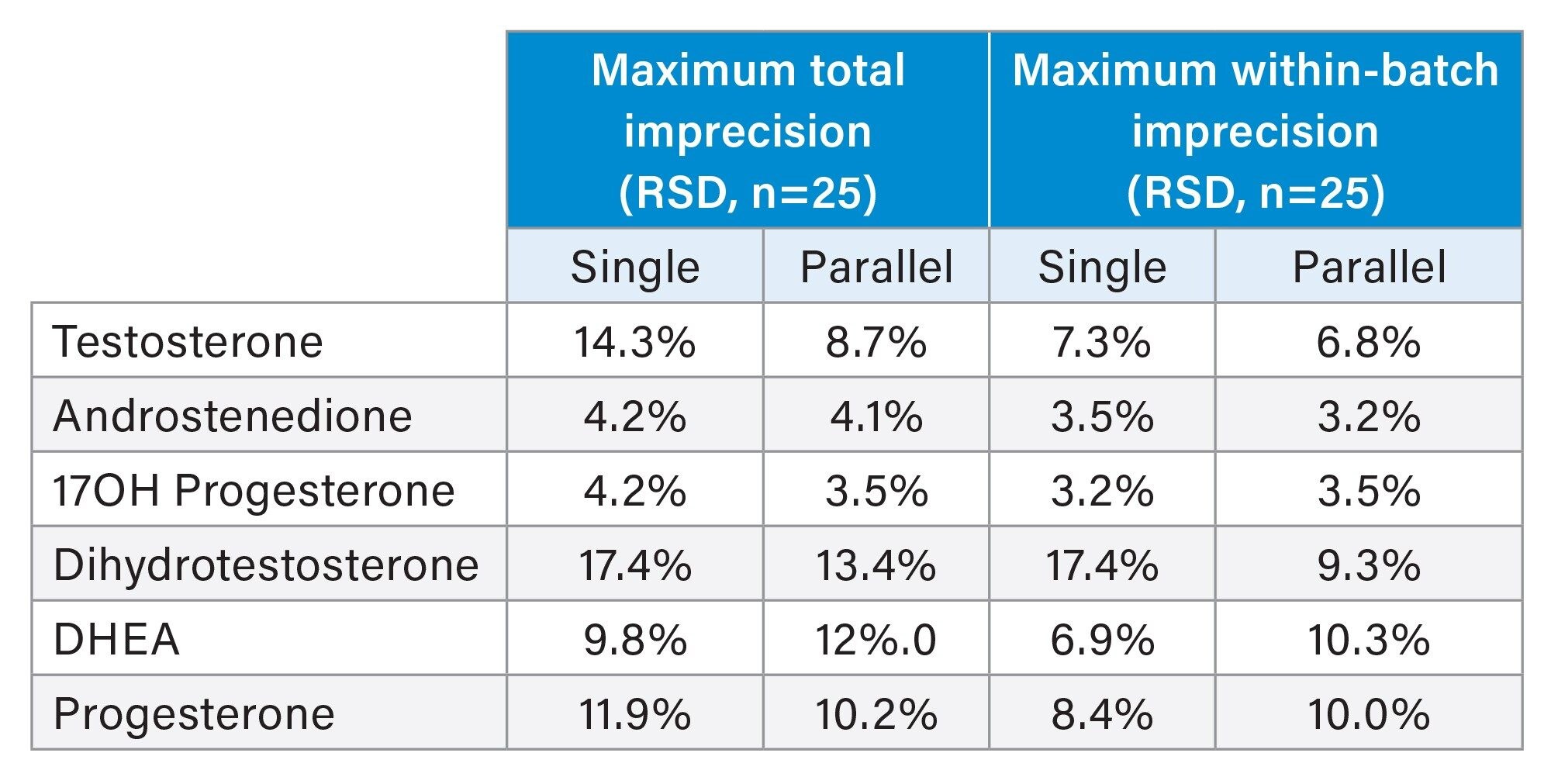
Single and parallel column imprecision was acceptable at ≤15% relative standard deviation (RSD), with the exception of single column analysis of dihydrotestosterone (DHT) in low concentration control samples. Interestingly, the within-batch and total precision was improved for this analyte at low concentrations, when using parallel column regeneration. A larger sample size is needed, however, to draw firm conclusions regarding the statistical significance of any effects on variance.
The maximum total and within-batch measurement imprecision with single and parallel column regeneration is summarized in Table 2.
Agreement between the analytical techniques was noted for all EQA samples analyzed (Figure 4).
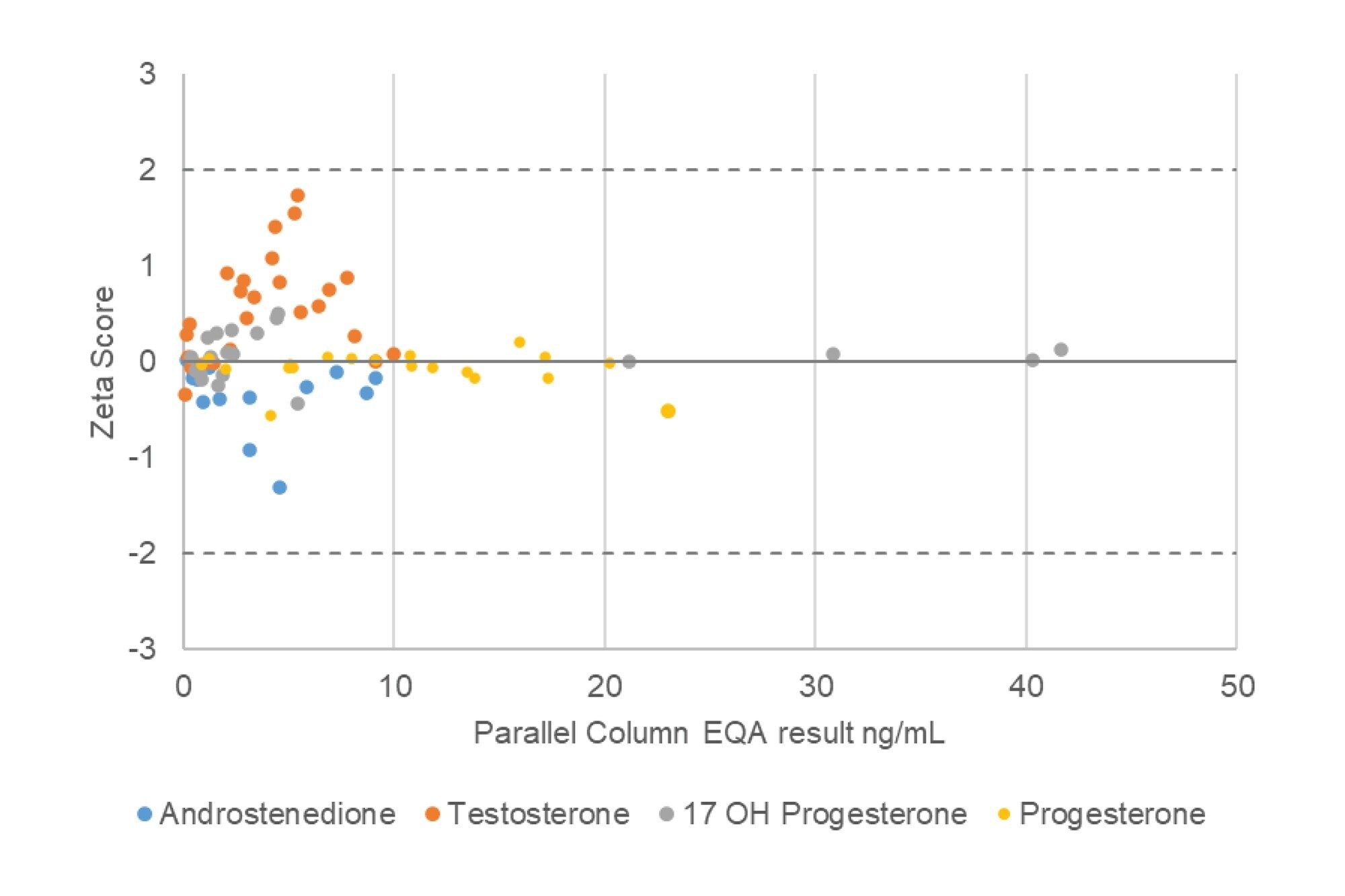
The results of EQA sample analysis suggested no significant quantitative differences between single and parallel column regeneration, and this was confirmed in the longer term, with the finding that mean control sample results made with parallel column regeneration were not significantly different to single column results (Student’s t-test, data not shown, p <0.05). Samples for DHEA EQA were not available for analysis.
Splitting calibrators between two LC columns gave similar results to those derived from a full set of calibrators analyzedanalysed using a single column. The potential to apply a single calibration across two LC columns presents an efficient and simplified acquisition and processing workflow.
Conclusion
Parallel column regeneration increased the efficiency of sample analysis without compromising column care and use best-practices. This technique may be of interest to any laboratory involved in the analysis of large numbers of clinical research specimens.
720008639, December 2024