
In this application note, we have investigated and present the results of multiple CCS applications, involving three ion mobility platforms – two Q-ToF travelling wave ion mobility mass spectrometry platforms and a cyclic ion mobility (cIM) research platform. The investigations included hundreds of analytes relevant to forensic toxicology for which a comparison has been performed where commonality in analyte investigation has occurred. Additionally, ion mobility investigations into classes that include veterinary drugs (fluoroquinolone protomers), medicinal plant extract analysis (flavonoids), pesticide screening, food analysis (steviol glycosides), and system performance monitoring (QC analytes) will be utilized to illustrate the robustness of routine collision cross section measurements.
Since the first coupling of mass spectrometry (MS) and ion mobility (IM) in 1962, there has been a continuous increase in the research and utility of the technique. The increase in IM-MS has been particularly evident over the last two decades where the number of peer-reviewed papers has increased from ~100 in 1995 to >1250 by 2014/2015, aided by the commercialization of IM instrumentation.1-4
UPLC-IM comprises ion mobility (gas phase separation prior to MS analysis) coupled with UPLC (neutral species separation). The timescale of UPLC (seconds), IMS (milliseconds), and time-of-flight MS (microseconds) are compatible with the requirements of high throughput analysis of complex samples. Ion mobility separation of compounds result from gas phase ions being separated within a gas-filled travelling wave ion mobility (TWIM) RF ion guide of the mass spectrometer, prior to the mass analyzer. Mobility separation is obtained by driving packets of ions through an inert buffer gas (typically nitrogen or helium) using a relatively weak electric field. The number of collisions between ions and the buffer gas cause drift time differences. The resultant separation is based on the application of repeating DC pulses along the RF ion guide. In this scenario, ions are periodically overtaken by the pulses or waves, and where less mobile species are overtaken more frequently than higher mobility species. Hence, the time to traverse the device is mobility-dependent on such factors as the mass, charge, and shape of the ion. It provides an added dimension of separation to that of LC (hydrophobicity) and MS (m/z), in addition to CCS (collision cross section), a complementary identification metric.
The continuing evolution of ion mobility has been illustrated where a strategy to incorporate ion mobility CCS values as an additional CCS cumulative metric to enhance specificity in pesticide screening assays was developed.5-7 The routine use of CCS for small molecule analysis has since increased across multiple areas of research including pharma (metabolism, metabolomics, lipids), food safety (veterinary drugs, mycotoxins, steroids, steviol glycosides, natural product screening, natural toxins). CCS searchable libraries have been generated, where use of a CCS metric can be used to increase cumulative specificity of identification as well decrease false detections.
We have investigated and present the results of multiple CCS applications, involving three ion mobility platforms – two Q-ToF travelling wave ion mobility mass spectrometry platforms and a cyclic ion mobility (cIM) research platform.
The cIM device in a modified SYNAPT provides a longer mobility separation path length and, consequently, higher mobility resolution than the standard linear TWIM cell. In addition, the multi-pass capability can provide significantly higher resolution over a reduced (selected) mobility range. The cIM device consists of a 100 cm path length RF ion guide comprising over 600 electrodes around which TWs circulate to provide mobility separation.8
The studies included an evaluation of the long-term reproducibility of CCS measurement (up to four years), TWIM CCS calibration robustness, intra/inter-site measurements, and cross-platform CCS reproducibility. The investigations included hundreds of analytes relevant to forensic toxicology for which a comparison has been performed where commonality in analyte investigation has occurred.9 Additionally, ion mobility investigations into classes that include veterinary drugs (fluoroquinolone protomers),10 medicinal plant extract analysis (flavonoids),11 pesticide screening,7 food analysis (steviol glycosides),12 and system performance monitoring (QC analytes) will be utilized to illustrate the robustness of routine collision cross section measurements.
Vials: LC-MS Certified Clear Glass 12 × 32 mm Screw Neck Total Recovery Vial, with Cap and Pre-slit PTFE/Silicone Septa, 1 mL Volume, (p/n: 600000671CV)
For the analysis of substances relevant to forensic toxicology, UPLC chromatographic separation was achieved using an ACQUITY UPLC HSS C18 Column (150 mm × 2.1 mm, 1.8 μm). A reversed-phase gradient was used, comprising Mobile Phase A (5 mM aqueous ammonium formate buffer adjusted to pH 3 with formic acid) and Mobile Phase B (acetonitrile with 0.1% formic acid).9
For all other presented applications,7,9-12 UPLC chromatographic separation was performed using a linear reversed-phase gradient of Mobile Phase A (acetonitrile containing 0.1% formic acid) and Mobile Phase B (water containing 0.1% formic acid), with both a conventional UPLC (ACQUITY UPLC I‐Class PLUS System used with an ACQUITY UPLC BEH C18 Column (100 mm × 2.1 mm, 1.7 μm) and a micro-flow UPLC system (ACQUITY UPLC M-Class System connected to a Waters ionKey separation device - iKey PCA BEH C18 130Å (50 mm × 150 μm, 1.7 μm)).
MS source parameters: Electrospray ionization (ESI), in both positive (ESI+) and negative (ESI-) modes were utilized with HDMSE acquisition. Mass ranges varied between 50–2000 Da. Varying ESI capillary voltages were applied. Analytes included forensic toxicology standards, steviol glycosides, fluoroquinolones, flavonoids, and small molecule QC standards. Full experimental details have previously been described.7,9-12
Instrument platform #1: Waters Vion IMS Q-ToF-MS and Instrument platform #2 (Waters SYNAPT G2-Si).
IM resolution: Platform #1 IM resolution ≈ 20 Ω/ΔΩ full-width half-maximum (FWHM) and Instrument Platform #2
IM resolution ≈ 40 Ω/ΔΩ (FWHM) default IMS screening parameters were utilised.
Mass resolution: Platforms #1 and #2 were 30,000 and 20,000 respectively FWHM (at m/z 556). The reference lockmass calibrant was leucine enkephalin (C28H37N5O7 (m/z 556.2766 for ESI+ and m/z 554.2620 for ESI-).
Calibration of the IM cell for TWCCSN2 calculations was performed using an IMS/ToF Calibration Kit (p/n: 186008113) (Waters Corp. UK).
The mass spectrometer was mass calibrated ESI+ at 60,000 resolution FWHM over an m/z range of 50–1000 using an IMS/ToF Calibration Kit (Waters Corp.). The reference lockmass calibrant was sodiated raffinose ([M+Na]+ m/z 527.1583). Ion mobility resolution was ≈ 65 Ω/ΔΩ (FWHM) for a single pass around the cIM device; resolution increases with the square root of the number of passes. Ion mobility parameters included: cIM T-wave velocity = 353m/s, T-wave pulse amplitude = 25 V and gas flows of 120/25 mL/min for the respective helium/IM cells resulting in an IM pressure of 2.7 mBar.
Chromatography software: MassLynx 4.1 SCN 916/924
MS software: MassLynx 4.1 SCN 916/924
Informatics: MassLynx data post-processed using UNIFI 1.92
The cIM-based instrument control and data acquisition were facilitated by in-house software comprising a novel web-based user interface and a modified version of MassLynx 4.1. CCS values were determined for single pass separation by using DriftScope 2.9 and manual calibration using the Waters standard calibration form.
Generation of CCS libraries and their application have been performed using three ion mobility platforms; two Q-ToF linear travelling wave ion mobility mass spectrometry platforms (Vion and SYNAPT) and a cyclic ion mobility (cIM) research platform. The cIM device is in a modified SYNAPT platform that provides a longer mobility separation path length, where the standard linear IM cell is replaced by a multi-pass cyclic IM cell for increased mobility resolution.
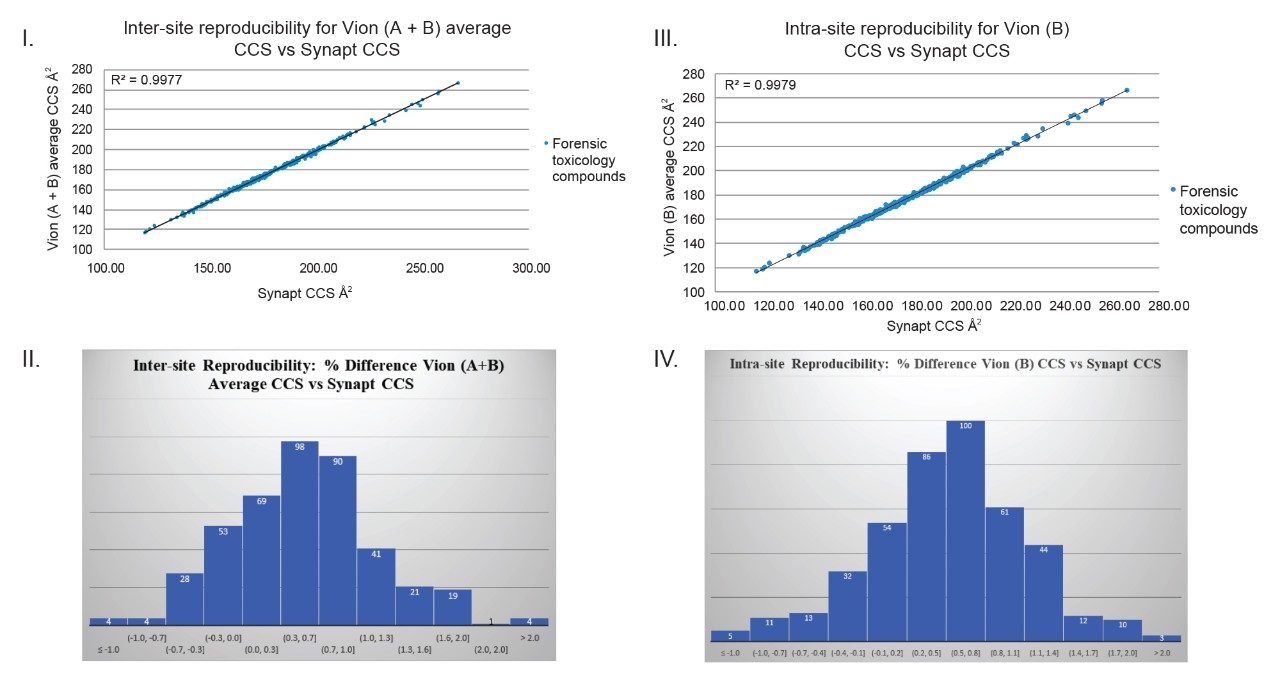
An extensive comparison of Q-ToF linear TWIM MS platforms has been performed. As part of an inter/intra-site study, a CCS library was generated for 500 forensic toxicology analytes, using Q-ToF TWIM MS instrument platform #1 (Vion A and Vion B), where replicate CCS measurements for a particular analyte were within 2% of each other. The average CCS values were used to create a forensic toxicology CCS library. This library was subsequently compared with a CCS library (for 600 forensic molecules) that had been generated using a SYNAPT in an independent study. A comparison of library contents revealed that 432 molecules had been analysed by both types of IM platform and significantly, that each of the 432 CCS values compared were found to be within 2% of each other; ~79% of the analytes compared had CCS values that were within 1% of the value measured using the alternative platform (Figure 1).
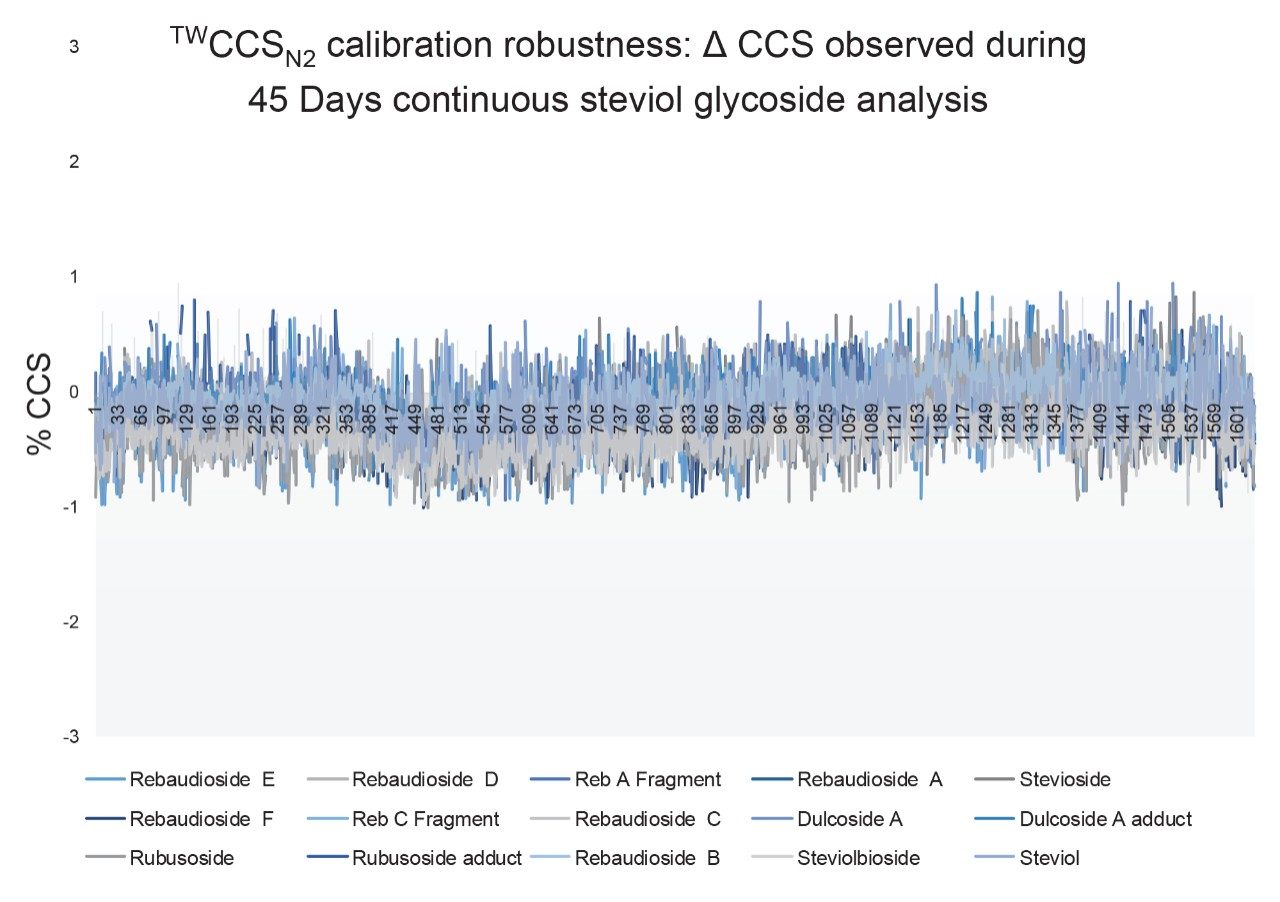
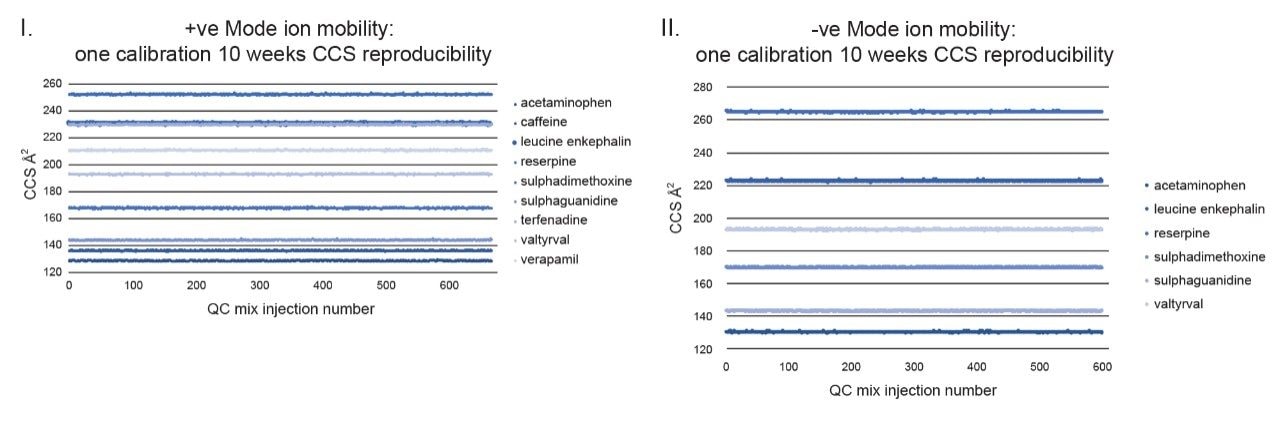
Although frequency of instrument calibration is often determined by individual laboratory protocols, Figure 2 illustrates the long-term stability of applying an ion mobility CCS calibration strategy. Using a steviol glycoside CCS library, a screening assay to detect steviol glycosides (E960) in extracts of ‘off the shelf’ complex food products such as jam, yogurt, soft drinks, and syrups was performed. Detection over three orders of dynamic range was achieved for the sweetener food additive E960. When compared to the reference library CCS Δ values <1% were achieved for 18974 detections with an RMS error of 0.26% over 45 days. A second example of applying a single CCS calibration, and hence CCS metric robustness, is presented where QC standards were monitored for a period of ten weeks in ESI+ and ESI- modes. During the process of building a small drug molecules CCS library, the positive (5360 measurements) and negative (3600 measurements) CCS Δ values of the QC standards were maintained within 2%, of the expected QC mix analyte CCS values as shown in Figure 3.
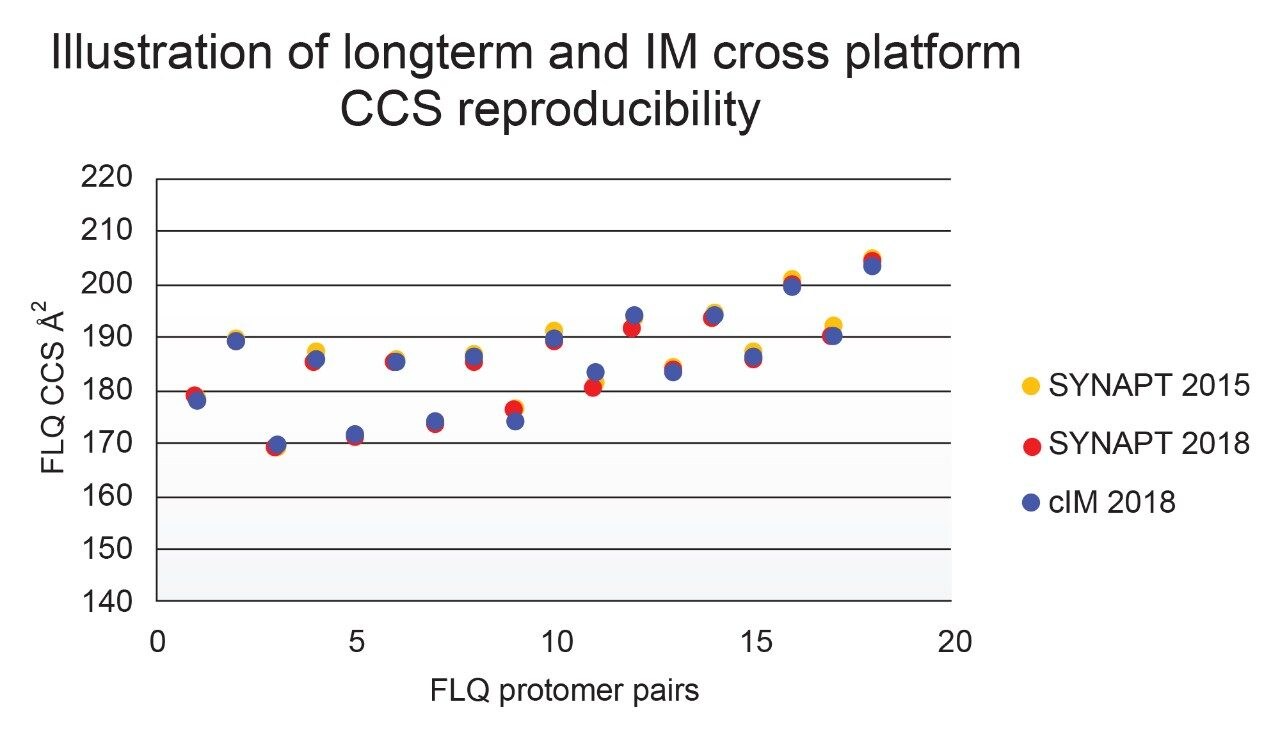
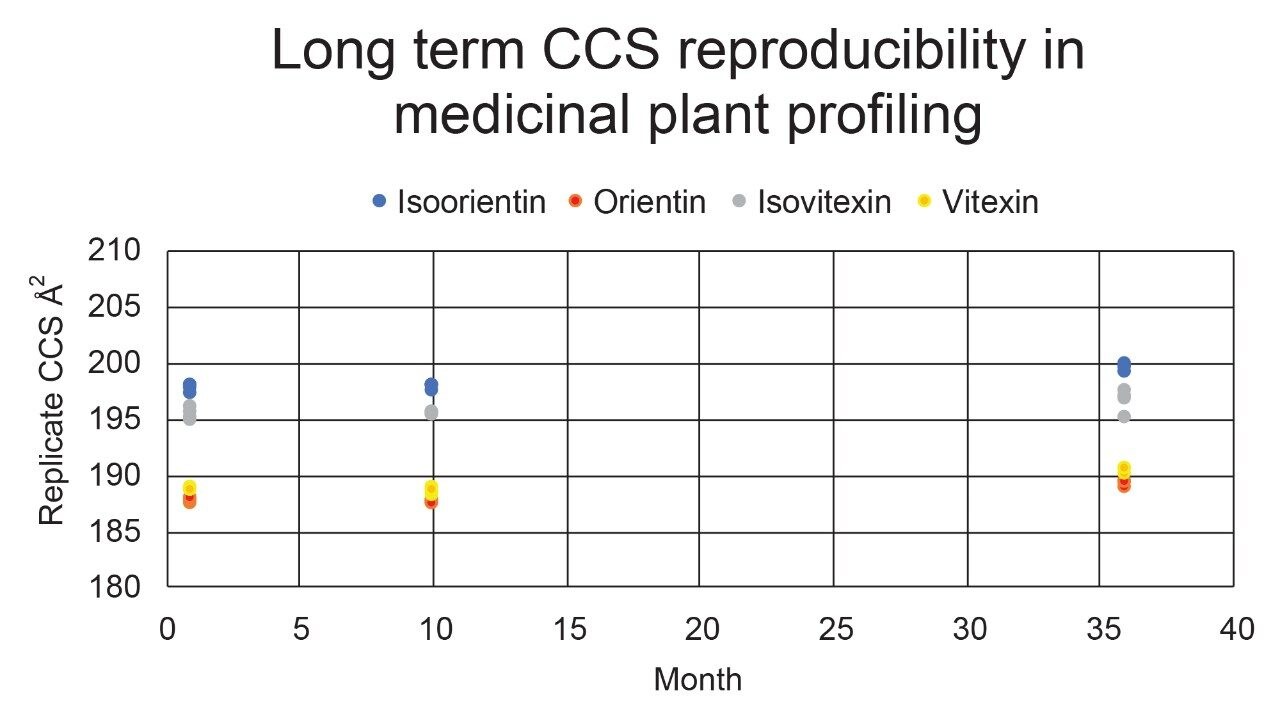
Using a fluoroquinolone veterinary drugs protomers study, CCS values were compared using Q-ToF TWIM MS instrument platform #2 and the multi-pass cIM device, where the observed CCS values were within 2% of the fluoroquinolone CCS library values. Figure 4 presents the cross-platform (linear IM and cIM) and long-term fluoroquinolone protomer CCS reproducibility over a three year period. When comparing linear IM and cIM CCS, Δ values <2% were obtained when compared to the fluoroquinolone protomer CCS library generated in 2015. Further long-term data is presented in Figure 5 in which identification of isomer marker flavonoids determined to be present in medicinal plant extracts of Passiflora species, where Δ values <2% were obtained when compared to the flavonoid CCS library generated in 2015.
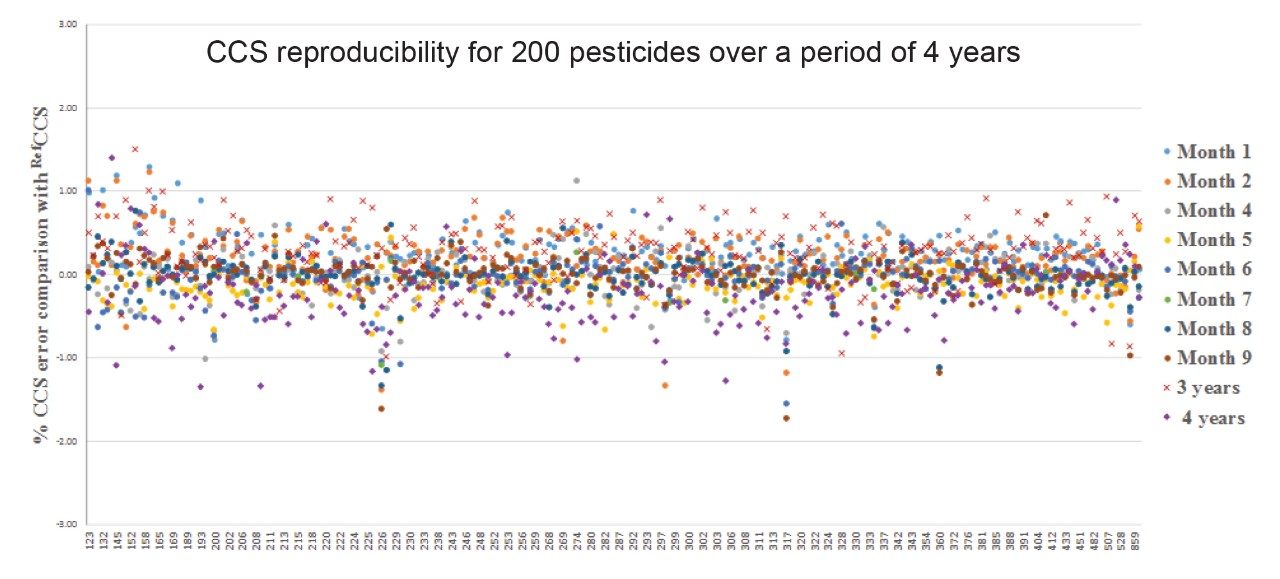
The extensive studies into TWIM CCS robustness are further illustrated in Figure 6, where the mean CCS errors (±SD) for the 200 pesticides comprising multiple classes of analytes have also been determined over a period of four years (2000 measurements), where CCS Δ <1% was obtained compared to the pesticide library generated in 2013. The library comprised a range of m/z 123 to 859 Da.
720006769, March 2020