
This is an Application Brief and does not contain a detailed Experimental section.
Azithromycin is a widely used antibiotic that has exhibited some success treating viral respiratory diseases. As a result of the COVID-19 pandemic, studies were initiated to investigate whether azithromycin, with or without other drugs, can help treat COVID-19 in different patient populations. Motivated by early clinical studies, Waters began investigating improved analytical methods for azithromycin. The currently approved USP monographs for azithromycin use mobile phases containing potassium phosphate buffers with various column chemistries ranging from typical L1 chemistries, to more exotic stationary phases. While the monographs are a useful starting point, implementing MS compatible mobile phases can provide increased versatility. The use of hydrophilic interaction chromatography (HILIC) complements the separations obtained using reversed-phase conditions.
Azithromycin, a widely used antibiotic, and other macrolide antibiotics such as clarithromycin, have shown some success in treating viral respiratory diseases including influenza.1,2 As a result, organizations initiated clinical studies to investigate azithromycin, with or without other drugs, as a potential treatment for SARS-CoV-2-infected patients.3,4 While some trials for azithromycin continue in certain COVID-19 patient populations, at least one trial studying azithromycin in combination with hydroxychloroquine in hospitalized patients has been cancelled.5
Even so, while organizations actively pursued clinical investigations into azithromycin during the early stages of the COVID-19 pandemic, we examined new analytical methods to support potential needs for improved analytical performance. Different analysis conditions were tested to best separate and characterize azithromycin. Azithromycin was successfully analyzed in HILIC mode using generic screening conditions and an ACQUITY UPLC BEH Amide Column. The result was narrower, more symmetrical peak shape and strong MS signal compared to common reversed-phase LC conditions.6 Such performance can be advantageous when analyzing low concentrations in bioanalysis applications.
Regardless of whether azithromycin is used in the treatment of the novel coronavirus, this new analytical method is still useful for azithromycin in general and may be transferrable in part for similar therapeutic molecules in need of low concentration analysis in bioanalysis applications.
Azithromycin was purchased from Sigma-Aldrich and diluted to 5 μg/mL in 95:5 acetonitrile:water. An ACQUITY UPLC BEH Amide Column (2.1 x 50 mm, 1.7 μm) was used with an ammonium formate (10 mM pH 3.0) mobile phase on an ACQUITY UPLC H-Class with a XEVO TQD Mass Spectrometer. The separation used the following gradient method. An isocratic hold of 95:5 acetonitrile:aqueous was performed for 1 minute, followed by a linear gradient to 70% aqueous in 7 minutes. The column was then re-equilibrated to starting conditions for the next injection. The mobile phase pH and ionic strength were kept constant throughout the gradient. A 1 μL injection, with a flow rate of 0.5 mL/min was used. The column temperature was set to 30°C. Azithromycin was detected using positive ion electrospray ionization with multiple reaction monitoring of the following m/z transitions: 749->591 and 749->158.4
The chromatogram below shows the results observed for azithromycin. A narrow peak was obtained, with excellent peak symmetry. Elution occurs at approximately 30% aqueous mobile phase under the described test conditions allowing for additional optimization of the method. Additionally, with such good retention, the peak does not elute with the unretained matrix compounds which often cause issues with MS sensitivity. Compared to the cited reversed phase method, the sharper peak achieved with this method facilitates lower detection limits, which is important for biological samples. Furthermore, the highly organic mobile phase used in this HILIC method could offer improved MS sensitivity compared to reversed-phase separations.
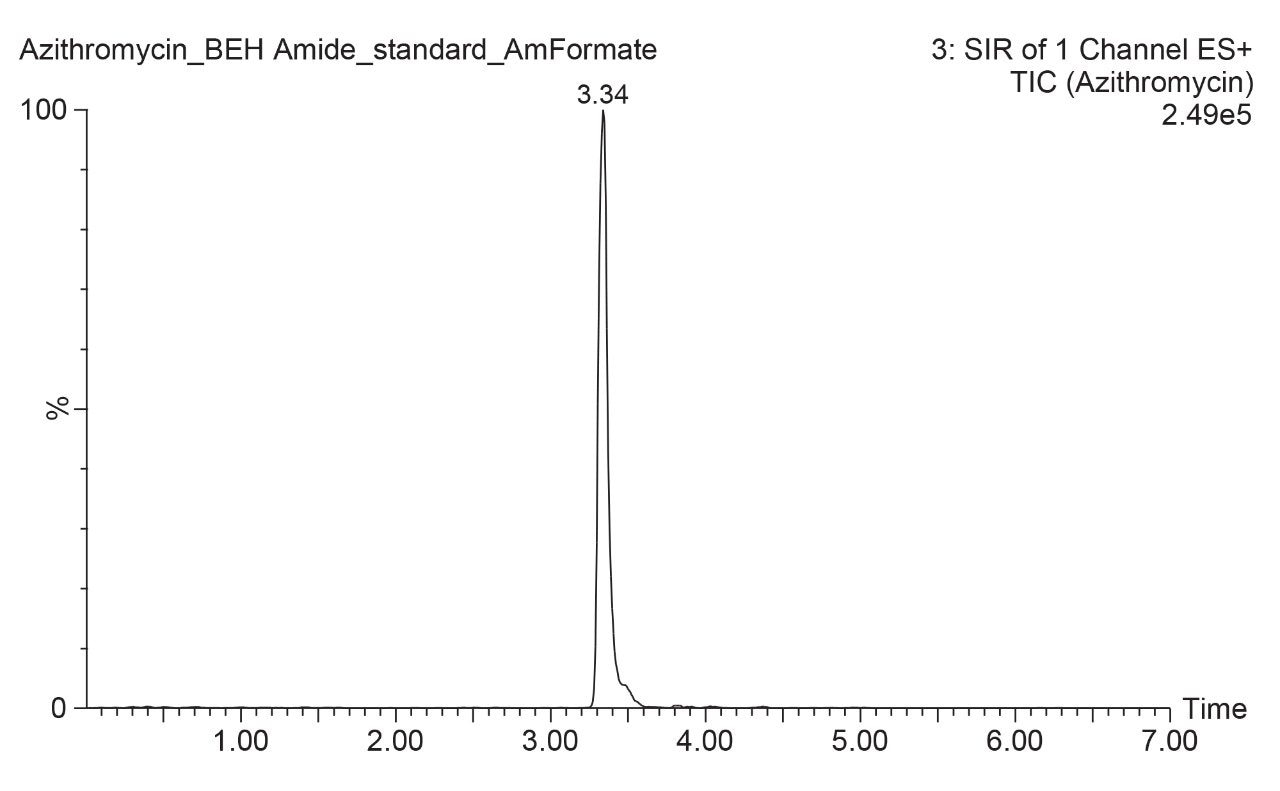
Reliable and robust analysis techniques for azithromycin are important in bioanalysis applications regardless of whether azithromycin turns out to be useful in treating COVID-19. While azithromycin has been successfully analyzed by reversed-phase LC, using HILIC provides several benefits. First, the good retention of this polar analyte in HILIC allows for method optimization, while reversed-phase applications are limited to mostly aqueous, or weak, mobile phases. Additionally, the high organic mobile phases used in HILIC promote better sensitivity in MS applications. This allows better detection of low-level analytes typically seen in bioanalysis workflows. Such bioanalytical workflows may become increasingly important as scientists continue to investigate azithromycin. HILIC can provide these benefits with minimal effort on the part of the analyst.
720006922, Revised July 2020