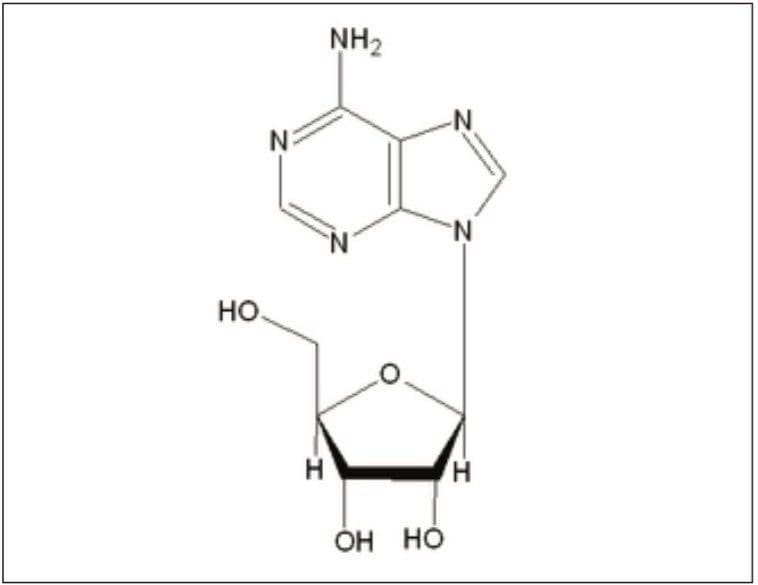
This application note summarizes the use of the Alliance HPLC System and IS Columns for a rapid and effective assay of adenosine injection USP.
Adenosine is an endogenous chemical involved in a wide range of metabolic processes, such as energy metabolism and inflammation.
At pharmaceutical doses, injected adenosine can effectively treat certain types of cardiac arrhythmias. With a half-life of less than 10 seconds in the blood, this is a fast-acting and fast-clearing drug.
Fast chromatography is a High Performance Liquid Chromatography (HPLC) technique using short columns packed with small particles to deliver rapid separation of simple mixtures that is desired. The Waters Alliance HPLC System with Waters Intelligent Speed (IS) Columns delivers excellent performance for fast chromatography. The Alliance HPLC System can perform an assay at higher flow rates and lower backpressures, increasing analytical throughput while meeting or exceeding assay acceptance criteria.
This application note summarizes the use of the Alliance HPLC System and IS Columns for a rapid and effective assay of adenosine injection USP.
|
LC system: |
Alliance e2695 Separation Module |
|
Column: |
Atlantis IS 4.6 x 20 mm dC18, 3μm |
|
Column temperature: |
40 °C |
|
Sample temperature: |
15 °C |
|
Injection volume: |
2 μL |
|
Mobile phase: |
10 mM sodium phosphate, 5 mM tetrabutyl ammonium phosphate, 2% acetonitrile in water (isocratic) |
|
Flow rate: |
3.0 mL/min |
|
Run time: |
2.5 min |
|
Detection: |
Waters 2998 Photodiode Array (PDA) Detector |
|
PDA wavelength: |
254 nm at 1.2 nm bandwidth |
|
Date rate: |
10 Hz, filter time constant: “fast” (0.1 s) |
The Alliance HPLC System was plumbed with 0.005” PEEK tubing pre-column and post-column to the 2998 PDA Detector to minimize band broadening. The sensitivity, suitability, adenosine standard, and sample test solutions were made according to the monograph for Adenosine Injection USP.1 The run time of the analysis was based upon two and a half times the retention time of the adenosine peak from the analysis of the assay sensitivity solution, as per the USP monograph.
The instrumental parameters for this Alliance HPLC System/IS Column assay developed from the original HPLC method. The original USP method called for a 3.9 mm x 30 cm, 10 μm L1 HPLC column, a flow rate of 2.5 mL/min, and an injection volume of 10 to 20 μL. Limited information concerning the original assay indicated a relatively short runtime, 6 minutes, with low retention and marginal resolution. A modern upgrade of the method was sought.
The short run-time and higher flow rate made this analysis conducive to an IS Column-based method. To that end, an Atlantis T3 IS 4.6 x 20 mm Column replaced the 3.9 x 300 mm HPLC Column, and the flow rate was increased to 3.0 mL/min, the classic IS Column flow rate. The 2 μL injection volume was determined by experiment to give the best sensitivity without causing column overload. These three simple changes delivered an assay that far exceeded the USP acceptance criteria (Table 1).
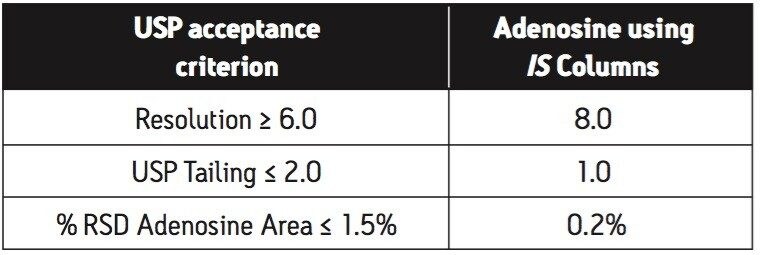
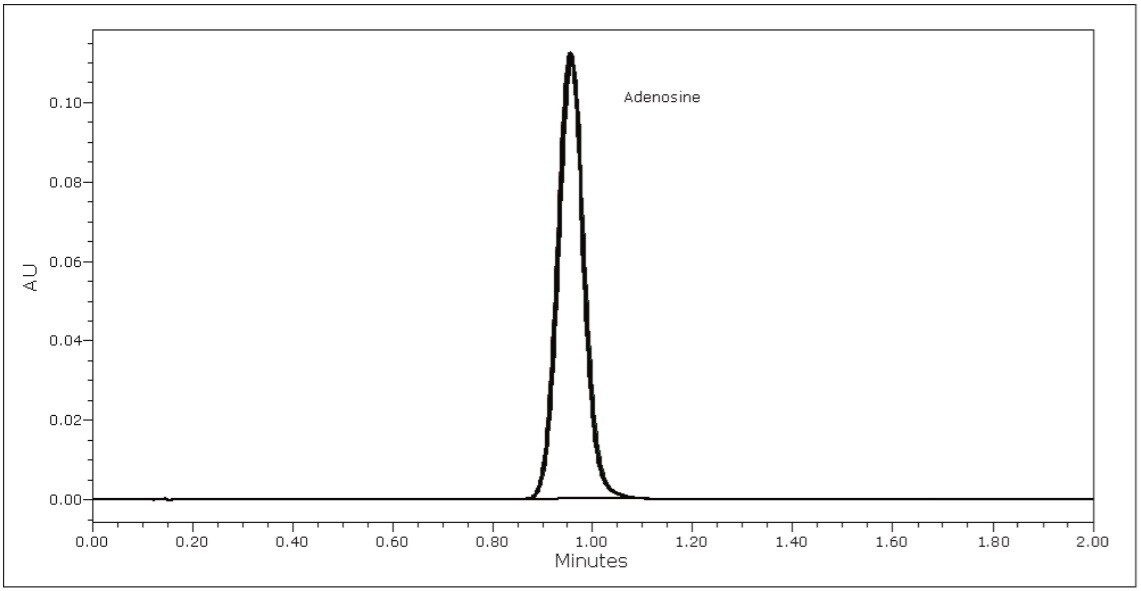
In overlay, the peaks of the assay suitability solution were well resolved and quite symmetrical (Figure 2). The nucleoside inositol was used here as a resolution standard. The mean USP resolution of the inositol and adenosine peaks was 8.0, much greater than the 6.0 required by the USP. The mean tailing factor of the adenosine and inositol was 1.0 and 1.1, respectively. Again, this was far superior to the acceptance criterion of 2.0. With a % RSD for area precision of 0.2%, this assay is highly repeatable. The retention time precision of 0.2% RSD was not reported in the table.

The overlay of six chromatograms of an assay test solution (Figure 3) also shows the excellent retention time and area repeatability of this analysis.
720002924, December 2008