Over two thirds of marketed recombinant biopharmaceutical products are glycoproteins, and regulatory agencies require the use of state-of-the-art glycan analysis methods for the development and commercialization of safe and effective glycosylated biotherapeutics.
Waters can help improve the quality of your glycan analysis and reduce sample prep time with easy to use workflow-based approaches, transforming what had once been a specialized and complicated activity into one in which scientists of any skill level can be successful. Our solutions span the entire development cycle— from released glycan monitoring and characterization, to glycoprotein profiling, middle-up/down, subunit analysis, monosaccharide and sialic acid composition, and glycopeptide mapping. Partner with Waters for the latest advancements to keep your lab compliant, accelerate your decision-making, and advance molecules to the clinic faster.

Demo: Glycan Analysis Workflow with Andrew+ Pipetting Robot

Streamline sample preparation for quantitative LC-MS analyses with verified workflows that help minimize variability, improve traceability, and simplify method transfer.
Accelerate the journey from sample analysis to decision-making with waters_connect for biopharmaceuticals that offers dedicated apps and workflows for MAM, intact mass analysis, sequence confirmation, and more.
Equip your lab with Empower Chromatography Data System (CDS) and gain advanced laboratory data management for your impurity analyses, including acquisition, processing, and reporting for liquid and gas chromatography instruments.
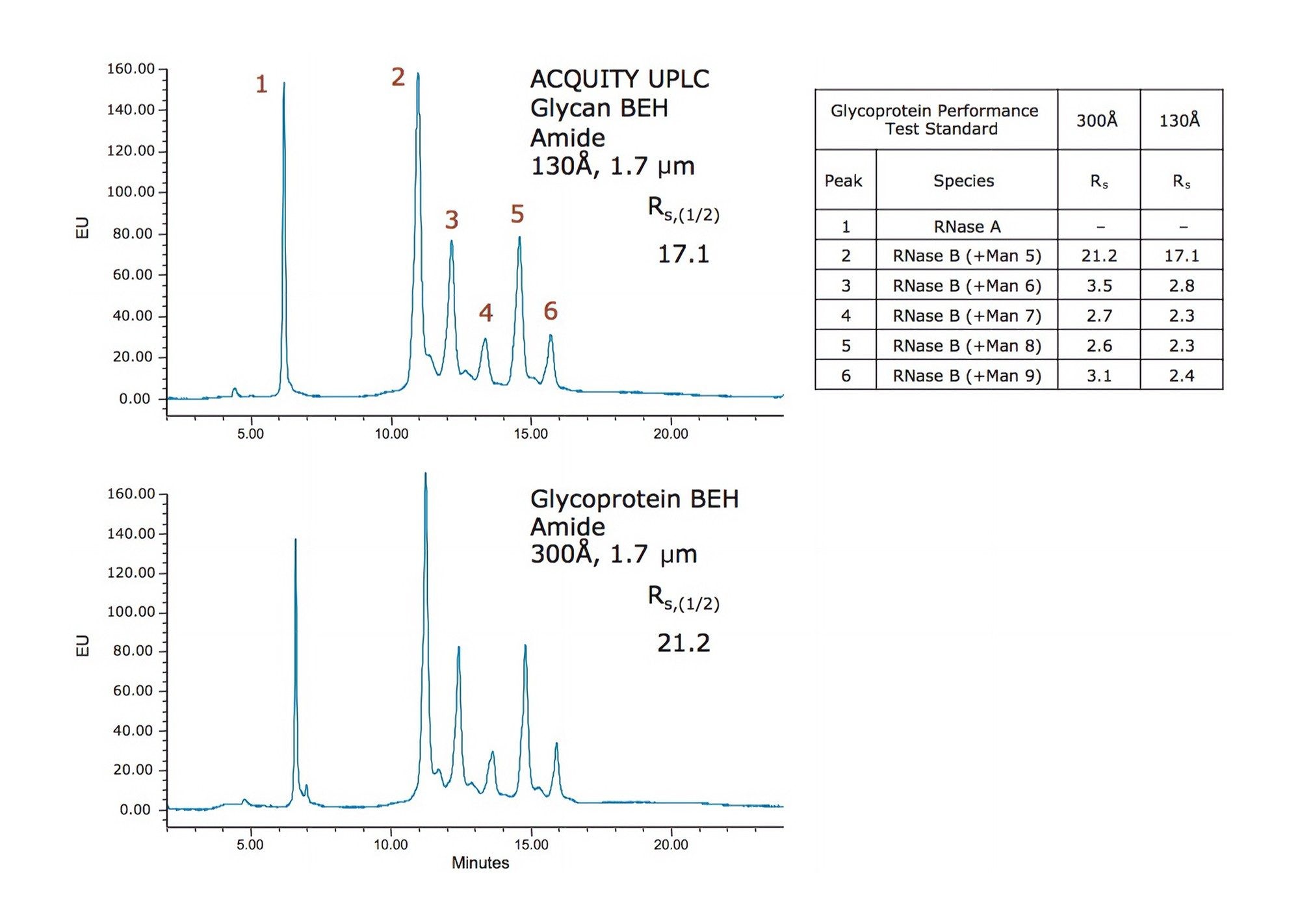
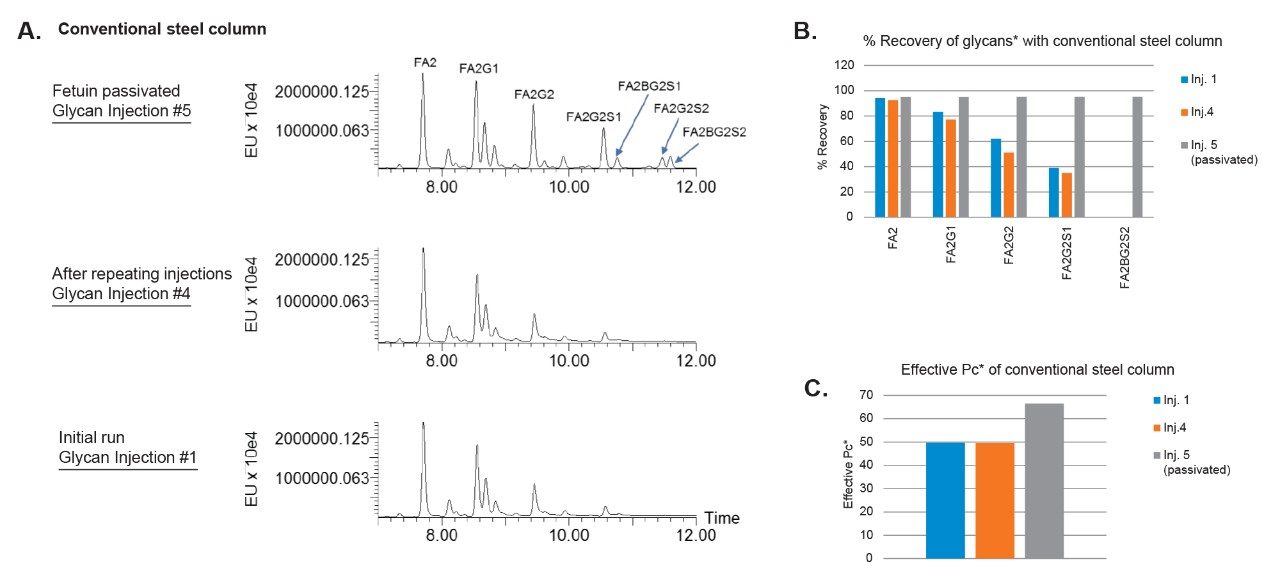
Obtain fast, high resolution glycan identification with Waters complete line of glycan columns to analyze glycoproteins, glycopeptides and released N-glycans with exceptional reproducibility.
Achieve unprecedented fluorescent and mass spectrometric performance for glycan detection with GlycoWorks N-glycan Kits that improve the throughput of N-glycan sample preparation so you don’t have to compromise between speed and sensitivity.
Verify your column performance upon receipt and monitor column condition on a regular basis with Waters Glycan Standards and run your glycan separations on any LC-MS system with ease and confidence.
Optimize your laboratory's productivity and success with Waters Global Services to maintain peak system performance, minimize down time, address application challenges, and support stringent compliance requirements.
Maximize resources and minimize risk with payment options from Waters Capital, including upgrading aging equipment, getting customized support, and bundling services into one monthly payment.