Absorptive losses have been known to occur with phosphorylated peptides using metal surfaces such as stainless-steel. While inert hardware such as PEEK-lined columns have been employed to minimize the interaction between acidic moieties and metal surfaces, a more pressure stable and widely applicable technology exists. MaxPeak Premier Columns employ a novel surface technology to greatly reduce, if not eliminate, binding of analytes to metal surfaces. The work shown here demonstrates the improved MS sensitivity of phosphopeptides using a single quadrupole MS detector that can be achieved by using MaxPeak Premier Columns.
Metal surfaces interact with acidic compounds through Lewis acid-base interactions that may lead to adsorption of the compounds onto the metal surfaces. This can prove detrimental to the analysis of these acidic compounds by LC and LC-MS as most systems and packed columns consist of metal hardware. Detrimental effects because of this non-specific binding include peak tailing and low analyte recoveries. While strategies exist to mitigate these interactions, like using a passivation agent or inert hardware such as PEEK, these methods pose their own issues, including decreased operating pressures or MS interferences.
MaxPeak High Performance Surfaces (HPS) Technology was introduced specifically to address the concerns of metal-analyte interactions without sacrificing operating pressure. MaxPeak Premier Columns include an organic/inorganic barrier that mitigates interactions between acidic analytes, such as phosphorylated probes, and chromatographic metal surfaces. This benefit has been documented for small molecule, oligo, and peptide separations.1 In addition, MaxPeak High Performance Surfaces (HPS) hardware does not require any special passivation solvents and is compatible with a wide range of mobile phases to allow for method development and optimization activities.
To demonstrate the benefits of MaxPeak Premier Columns for LC-MS analysis, the MassPREP Phosphopeptide Standard Enolase was analyzed on three columns using an ACQUITY UPLC H-Class System coupled to an ACQUITY QDa Mass Detector for MS detection. The XSelect Peptide CSH C18 Column and a competitor peptide column was compared against an XSelect Premier Peptide CSH C18 Column with MaxPeak HPS Technology. A phosphopeptide standard was chosen as they are generally less abundant than non-phosphorylated peptides and are known to adsorb to metals. Additionally, the accurate analysis of these peptides is critical for many workflows. Comparisons between all three columns were made regarding analyte recovery through peak areas and peak heights. Here, the XSelect Premier Column provided the highest sensitivity and recovery for all phosphorylated peptides, leading to increased reproducibility from injection to injection.
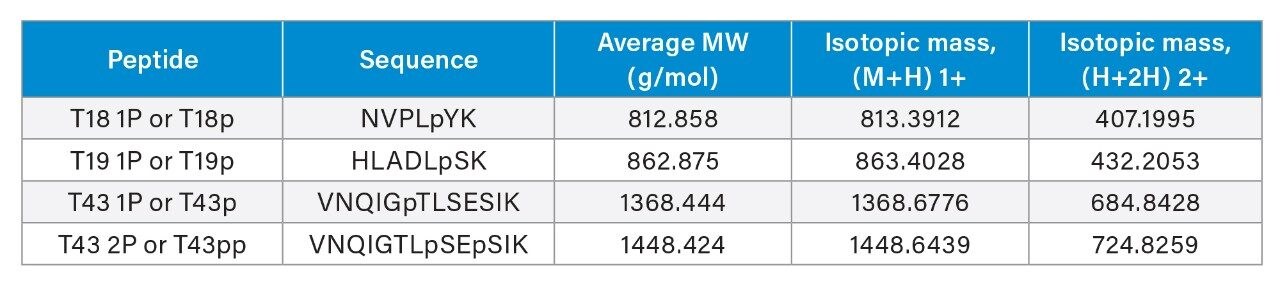
The MassPREP Phosphopeptide-Enolase standard (PN: 186003285) contains 1 nmol each of four synthetic enolase phosphopeptides: T18 (1P, tyrosine), T19 (1P, serine), T43 (1P, threonine), and T43 (2P, serine). The vial contents were reconstituted in 50 µL Milli-Q water to give a 20 pmol/µL solution and vortexed for 10 seconds prior to injection.
|
LC Conditions |
|
|
LC system: |
ACQUITY UPLC H-Class System |
|
Detection: |
ESI+ Full Scan |
|
Vials: |
TruView LCMS Certified Clear Glass Vial Total Recovery (PN: 186005669CV) |
|
Column(s): |
XSelect Peptide CSH C18 XP Column, 2.1 x 50 mm, 2.5 µm (PN: 186006941) XSelect Premier Peptide CSH C18 Column, 2.1 x 50 mm, 2.5 µm (PN: 186009904) Competitor Peptide Column, 2.1 x 50 mm, 2.7 µm |
|
Column temp.: |
60 °C |
|
Sample temp.: |
Ambient |
|
Injection volume: |
1 µL |
|
Flow rate: |
0.45 mL/min |
|
Mobile phase A: |
0.1% formic acid in water |
|
Mobile phase B: |
0.1% formic acid in acetonitrile |
|
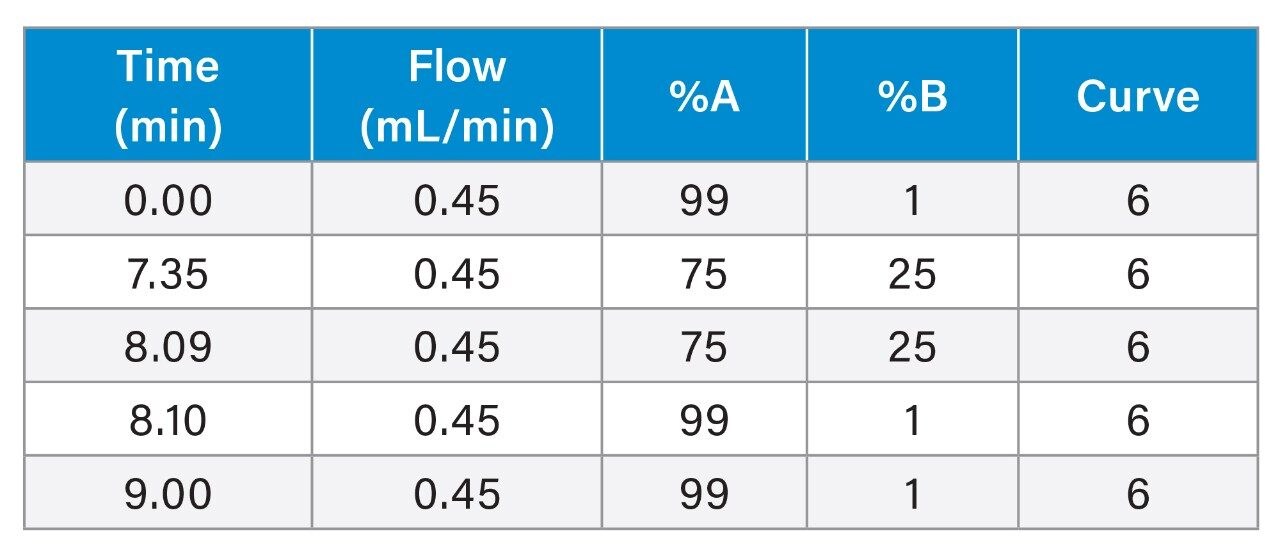
Gradient: |
See Table |
|
Chromatography software: |
Empower 3 Feature Release 4 |
|
MS software: |
Empower 3 Feature Release 4 |
|
Informatics: |
Empower 3 Feature Release 4 |
To demonstrate the benefits of MaxPeak Premier LC Columns, we evaluated the separation and recovery of a phosphopeptide standard using a conventional XSelect Peptide CSH C18 Column, a competitive peptide column, and an XSelect Premier Peptide CSH C18 Column. Figure 1 shows the extracted ion chromatograms of the first injection for each column. MS detection was performed using an ACQUITY QDa Mass Detector at the (M+2H)+2 mass indicated in Table 1.
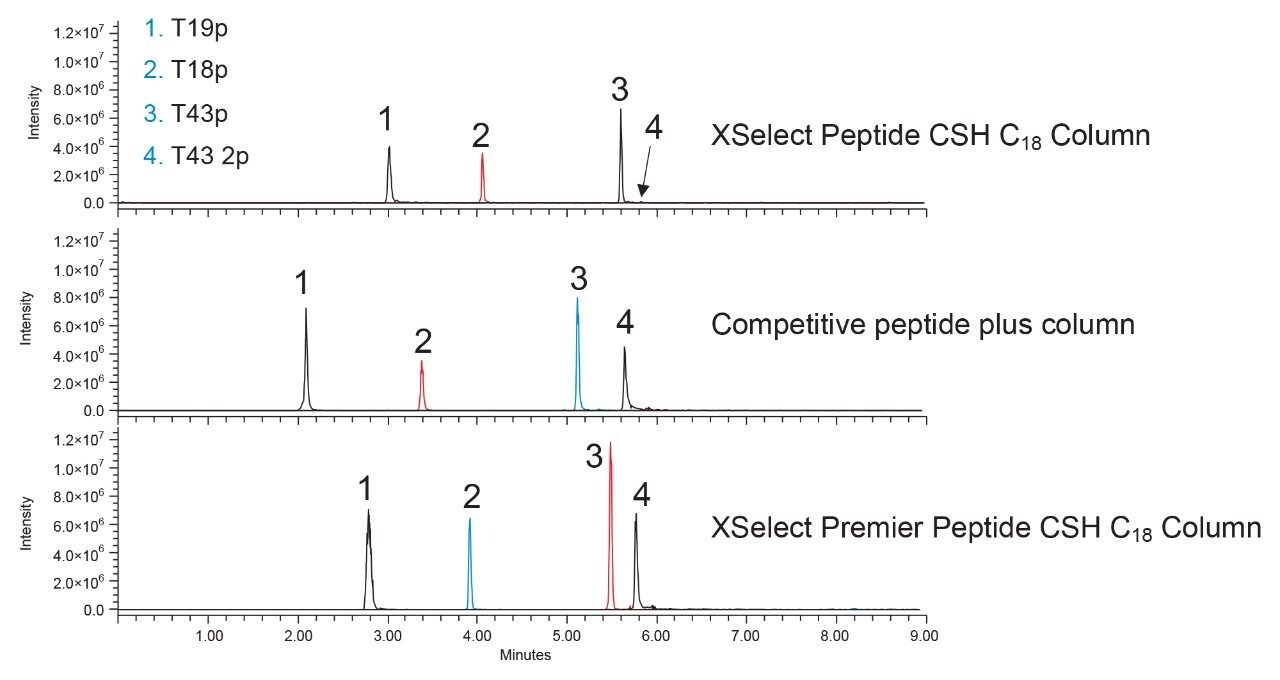
The XSelect Peptide CSH C18 Column produces the lowest signal intensity for all peptides, with the T43 2p peak almost undetectable from background noise and considerably lower in intensity compared to the singly phosphorylated peptides. The doubly phosphorylated peptide is more difficult to recover due to its extra acidic phosphate group and shows increased signal intensity using the competitive peptide column. However, the highest signal intensity achieved for T43 2p was with the XSelect Premier Peptide CSH C18 Column. In fact, all probes have higher signal intensities using XSelect Premier Peptide CSH C18 Column compared to the other columns tested. The singly phosphorylated peptides, though less acidic than the doubly phosphorylated T43 2p, still show adsorptive losses as indicated with lower signal intensities and peak areas for the conventional XSelect Peptide CSH C18 and competitor peptide columns (Table 2). Apart from the differences in signal intensity, the XSelect Premier Peptide CSH C18 Column achieves similar selectivity compared to the XSelect Peptide CSH C18 Column, especially for the T43p and T43 2p peaks. The competitive peptide plus column shows a better separation of those two probes but is overall less retentive than the XSelect CSH C18 chemistries, with or without the MaxPeak HPS hardware. A slight difference in retention is seen between the XSelect Premier Peptide CSH C18 Column and the XSelect Peptide CSH C18 Column with the standard hardware column showing slightly higher retentions, likely due to the ionic interactions between the probes and the metal surfaces. Being able to switch from a standard column to a MaxPeak Premier Column without major selectivity differences allows for faster adoption of MaxPeak HPS Technology in critical assays.
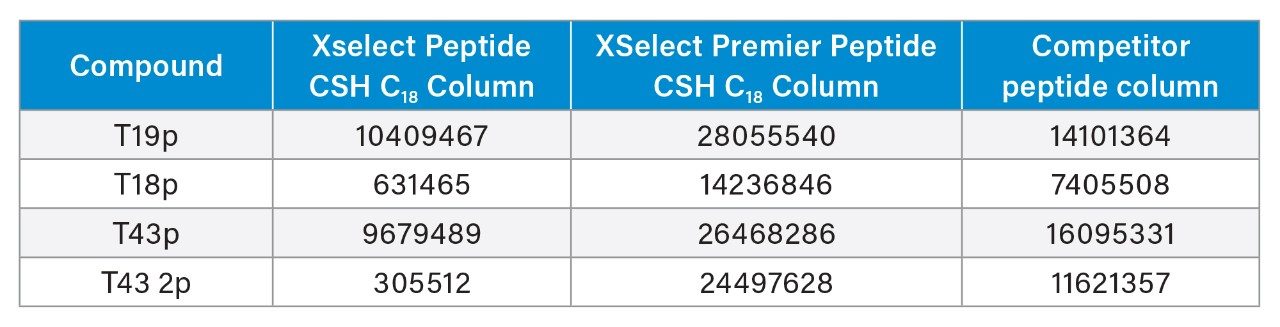
The most striking difference when considering peak area is again with the T43 2p peptide (Table 2) versus the MaxPeak Premier Column, upon first injection, peak areas for the T43 2p peptide were ~50% lower on the competitor peptide column and ~99% lower on the XSelect Peptide CSH C18 Column. However, when running successive injections, the peak areas for all phosphopeptides using both the conventional XSelect Peptide CSH C18 and competitor peptide columns begin to increase, as shown in Figure 2.
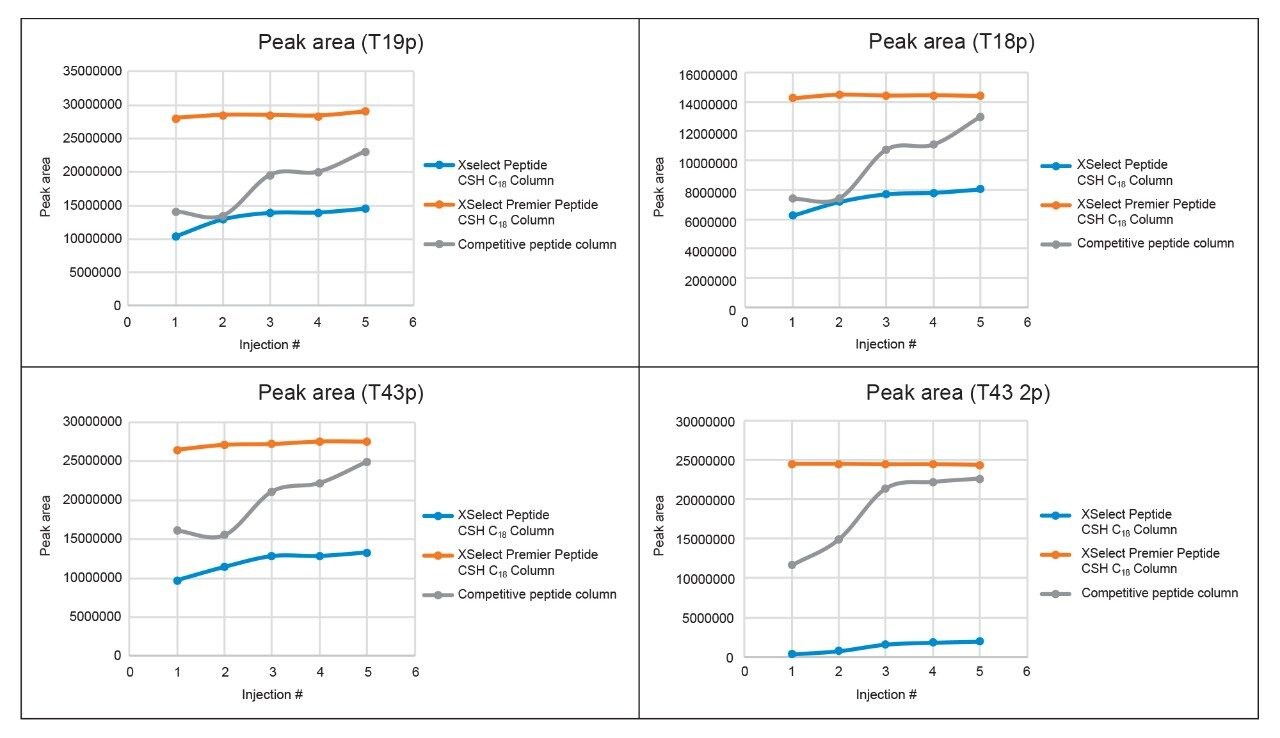
In Figure 2, the XSelect Premier Peptide CSH C18 Column yields reproducible peak areas from the first injection, in contrast to the other two columns. The XSelect Peptide CSH C18 Column demonstrates a very slow increase in peak area over five injections while the competitive peptide column improves more dramatically, but without a linear increase over time. In both cases, neither column reaches the performance of the MaxPeak Premier Column for any of the phosphopeptides within five injections, suggesting that either further column conditioning is needed or that the level of performance achieved with MaxPeak Premier Columns cannot be attained. The enhanced recovery of MaxPeak Premier Columns demonstrates the importance and utility of inert chromatographic surfaces for phosphopeptide analysis.
With MaxPeak Premier Columns, metal interactions between acidic analytes such as phosphopeptides and chromatographic surfaces have been drastically reduced. As demonstrated in this application note, using MaxPeak Premier LC Columns can improve the recovery of phosphopeptides for detection using single quadrupole mass spectrometers without significant changes in selectivity or analyte retention. The increased sensitivity using the XSelect Premier CSH C18 Column can allow for lower levels of peptides to be detected, granting better characterization of biological systems or protein digestions via peptide mapping. MaxPeak Premier Columns could thus be very useful when analyzing low concentrations, especially for low abundant phosphorylated peptides that often need enrichment prior to analysis.
720007292, June 2021