This application note explains important considerations made during the design and development of this mobile phase as well as some strategies that can be employed to optimize separations, whether it be using alternative mobile phase dilutions or the fine tuning of gradients.
A novel salt-mediated pH-gradient, ion-exchange (IEX) method is demonstrated that employs volatile salts to allow for direct coupling to a mass spectrometer and the straightforward and simple identification of chromatographic peaks.
Microheterogeneities are inherent features of monoclonal antibodies (mAbs) due to their susceptibility to both chemical and enzymatic modifications. Many of these modifications cause changes in surface charge, including deamidation, glycation, C-terminal lysine truncation, and some types of oxidation.1 Because these heterogeneities can impact efficacy and safety, it can become very important to have cutting-edge approaches for their detection. These so-called charge variant analyses can be performed using a variety of different techniques, but it seems that more and more promising methods are being developed using ion exchange chromatography (IEX).
Previously, IEX was seen as incompatible with mass spectrometry due to the use of high levels of non-volatile salts.2 To achieve peak identification, this incompatibility has required long workarounds such as fraction collection3 or cumbersome 2D-LC setups.4,5 Recent advances using relatively low concentrations of volatile salts have, however, allowed the hyphenation of IEX to MS and the direct identification of charge variants.2,6-8 Most notably, ammonium salts are now being used for IEX-MS mobile phases, and with them, it has become possible to achieve MS-compatibility with both salt and pH gradients.
Along these lines, we have developed a flexible ammonium acetate based, salt-mediated pH gradient technique suitable for the charge variant analysis of a wide range of mAbs. The developed mobile phase is based on the IonHance CX-MS pH Concentrates that yield solutions for performing binary pH gradients and collecting high sensitivity mass spectra. In the following work, we will explain important considerations made during the design and development of this mobile phase as well as some strategies that can be employed to optimize separations, whether it be using alternative mobile phase dilutions or the fine tuning of gradients.
100 μg of NIST mAb (NIST RM 8671), trastuzumab or adalimumab was digested by incubating at 37 °C for 30 min with 20 units of FabRICATOR enzyme (Genovis, A0-FR1-008) in either 105 mM ammonium acetate pH 6.2 or phosphate buffer saline. The final concentration of digested antibodies was 1.0 mg/mL. More information on considerations for using ammonium acetate versus phosphate buffered saline can be found in the application note “Practical Considerations for Optimizing MS Quality during IEX-MS” (p/n: 720006675EN).
|
LC system: |
ACQUITY UPLC I-Class |
|
Detectors: |
Xevo G2-S QToF |
|
LC column: |
ACQUITY UPLC Protein BEH SEC 200 Å, 1.7 μm, 4.6 × 150 mm (p/n: 186005225) |
|
Column temp.: |
30 °C |
|
Flow rate: |
0.100 mL/min |
|
Injection: |
5 μL (10 mg/mL NIST mAb – RM 8671) |
|
Mobile phase: |
50 mM ammonium acetate pH 5.0, 7.0, and 9.0 50 mM, 150 mM, and 300 mM ammonium acetate pH 7.0 |
|
Capillary voltage: |
3.0 kV |
|
Sampling cone voltage: |
150 V |
|
Source temp.: |
135 °C |
|
Desolvation temp.: |
500 °C |
|
Cone gas flow: |
300 L/h |
|
Desolvation gas flow: |
800 L/h |
|
LC system: |
ACQUITY UPLC I-Class PLUS |
|
Detectors: |
ACQUITY TUV ACQUITY RDa MS GE Healthcare Monitor pH/C-900 |
|
LC column: |
BioResolve SCX mAb, 3 μm, 2.1 × 50 mm (p/n: 186009054) |
|
Column temp.: |
30 °C |
|
Sample vial: |
LCMS Certified Glass Screw Neck Total Recovery Vials (p/n: 600000671cv) |
|
Mobile phase A: |
IonHance CX-MS pH Concentrate Buffer A pH 5.0 (p/n: 186009280) |
|
Figures 2 and 4A: |
50 mM ammonium acetate pH 5.0 |
|
Figures 3B, 4, 5, and 6: |
10 mM ammonium acetate pH 5.0 |
|
Mobile phase B: |
IonHance CX-MS pH Concentrate Buffer B, pH 8.5 (p/n: 186009281) |
|
Figures 2 and 4A: |
160 mM ammonium acetate pH 8.5 |
|
Figures 3B, 4B, 5, and 6: |
75 mM ammonium acetate pH 8.4 |
|
Figure 4A: |
53 mM ammonium acetate pH 8.4 |
|
Gradient table (Figures 1, 3, 4A, 4B, 5A): |
(Figure 2: 0.200 mL/min flow rate) |
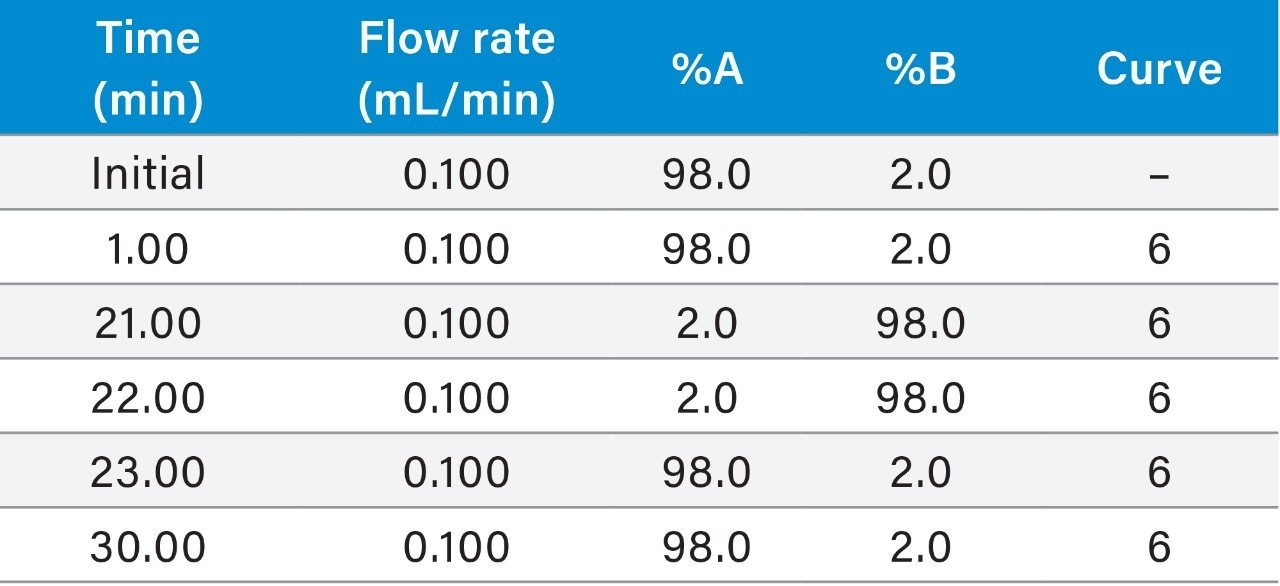
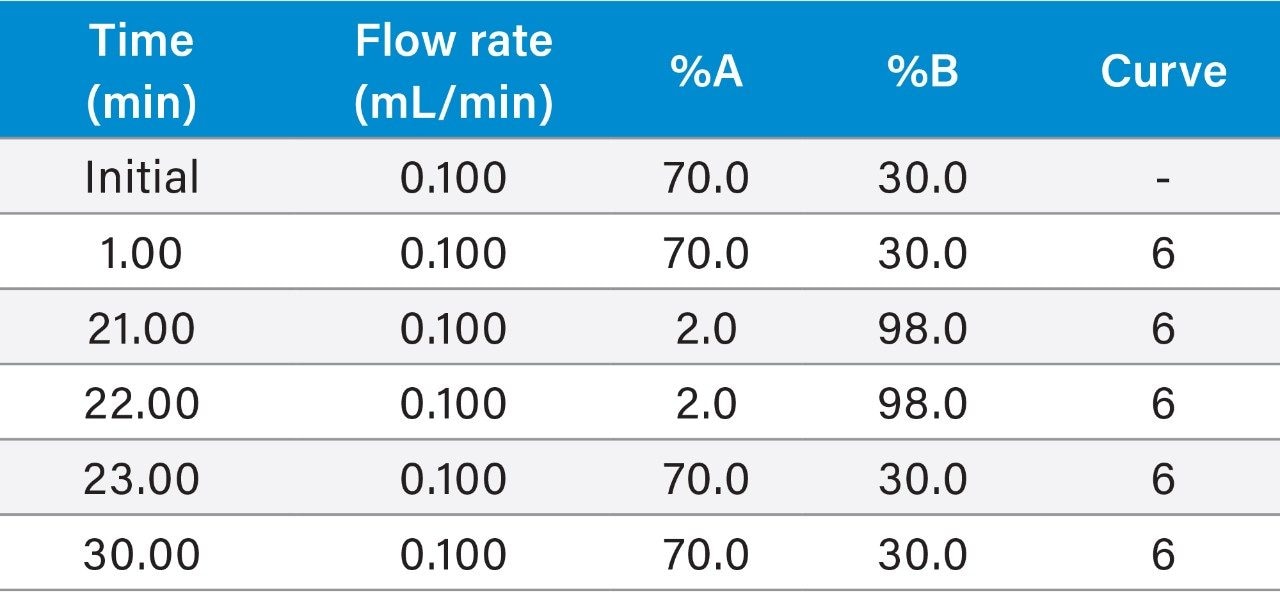
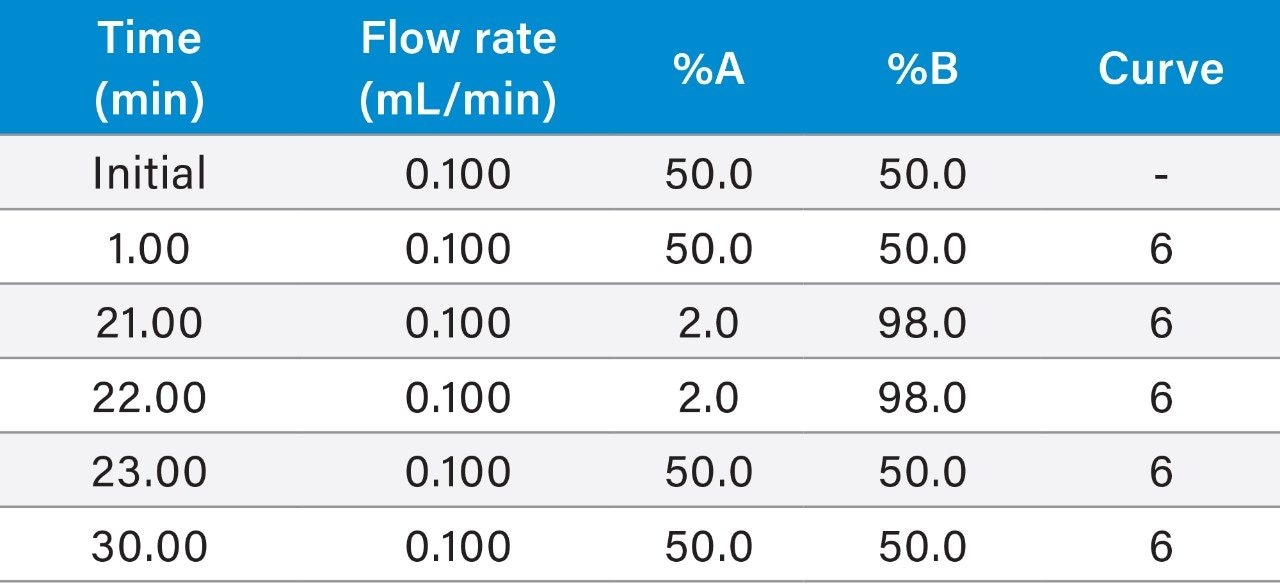
|
Mass range: |
m/z 400–7,000 |
|
Mode: |
ESI+ |
|
Cone voltage: |
150 V |
|
Desolvation temp.: |
350 °C |
|
Capillary voltage: |
1.5 kV |
|
Lock mass: |
Leu-enkephalin at 50 fmol/μL in 50/50 water/acetonitrile with 0.1% formic acid |
|
Informatics: |
UNIFI Scientific Information System |
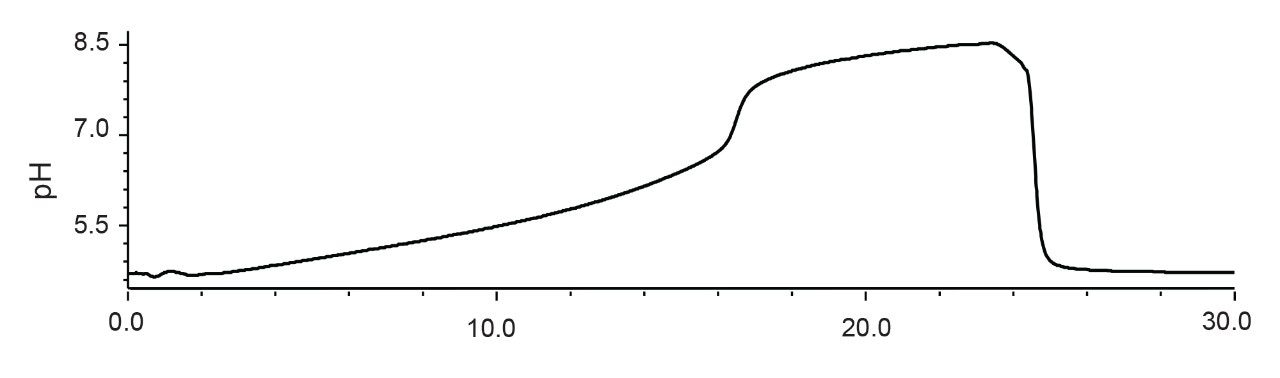
Recent advances in directly coupling ion exchange (IEX) to mass spectrometers (MS) have successfully allowed for the straightforward and simplified analysis of charge variants. This hyphenation has considerably reduced prep time for making MS-based identifications and is opening up opportunities for a new approach to monitoring the critical attributes of a drug product. The IonHance CX-MS pH Concentrates were created to address the challenges in adopting IEX-MS, namely the laborious nature of creating appropriate mobile phases. The IonHance CX-MS pH Concentrates are pH-stable, ammonium acetate solutions that are prepared from and packaged in materials with low ppb, trace metal certified materials. To ensure a long shelf life, the concentrates are also manufactured with 20% acetonitrile (ACN), which ensures they remain bacteriostatic until dilution. The suggested starting point for using these concentrates is a 10x dilution, which yields a 50 mM ammonium acetate pH 5.0 2% acetonitrile (ACN) solution and a 160 mM ammonium acetate pH 8.5 2% ACN solution for use as mobile phase A and B, respectively. With these mobile phases, it is possible to achieve a linear pH gradient during the elution window of mAbs (Figure 1). This is despite the pKas of acetate and ammonium being 4.75 and 9.25, because sufficient ionic strengths are employed throughout a run to lend buffering capacity and pH control (e.g., 10 to 200 mM concentrations).9,10 The following sections provide more information on how this composition came to be formulated as well as some examples on how to optimize a separation beyond the use of the standard 10x diluted concentrates.
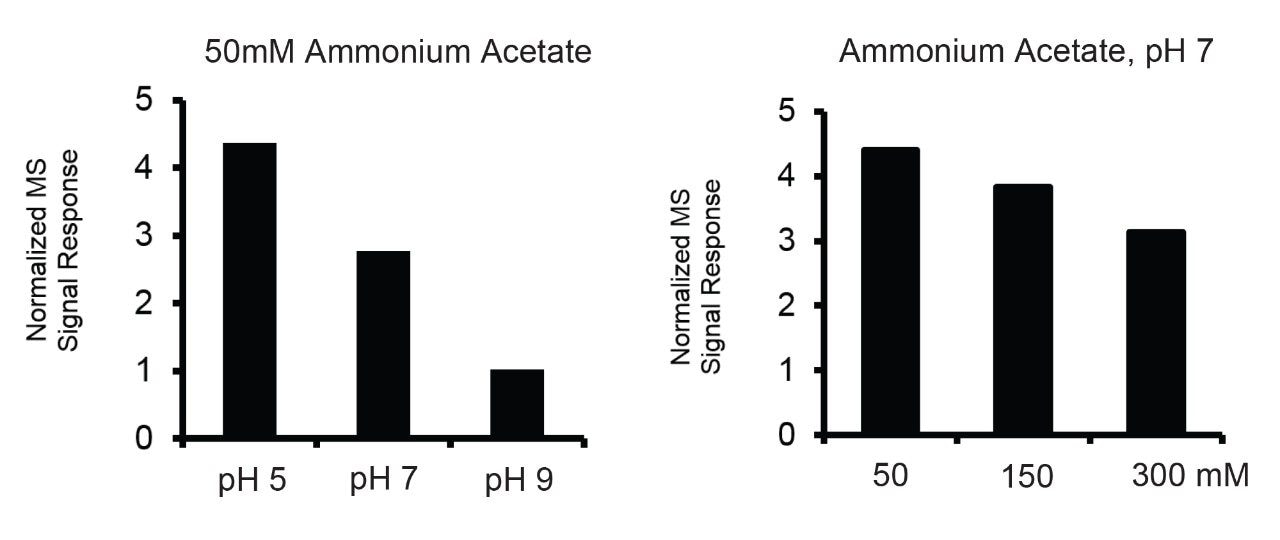
Mobile phases used for IEX-MS have included ammonium formate,7,11 ammonium acetate,7, 11,12 and ammonium bicarbonate.6,12,13 There is reason to give some thought to selecting the ammonium counter ion. Most particularly, analysts should re-consider the use of ammonium bicarbonate (or even carbonate), because it can cause proteins to denature during electrospray, the formation of CO2 adducts, and supercharging.10,14,15 While supercharging may be desirable in some circumstances, it can also complicate data analysis by creating MS signal with little to no correspondence to UV or fluorescence based chromatograms. The in situ formation of carbon dioxide can also cause problems by introducing +44 Da gas phase adducts. Moreover, bicarbonate solutions exhibit poor stability; bicarbonate solutions off-gas CO2 and shift in pH such that it is unreliable to keep them for any type of long-term storage. All of this culminates in more charge states, additional adducts, loss of native conformations, and poor mobile phase stability. Meanwhile, mobile phases comprised of ammonium formate suffer from disparate pKa values and comparatively low buffering capacity across commonly explored pH values. For these reasons, ammonium acetate is seen to be the best mobile phase component for native LC-MS experiments.
Differing methodologies have been used for IEX-MS, including pH gradients,11 salt gradients,7 and salt-mediated pH gradients.12,16 All have been shown to be compatible with native protein analysis; however, differences do exist between the methods. Salt gradients have generally been found to give higher peak capacities and greater resolving power than pH gradients1,17 but they pose a heavy burden on MS instrumentation. Moreover, impurities in high ionic strength buffers can yield unwanted metal adducts and diminish MS quality. A pH gradient, in contrast, affords elution by means of changing mobile phase pH.18 A protein’s net charge is modified during the gradient until the protein comes to elute from the column.18 To its advantage, a pH gradient method does not require high ionic strength and can generally be used more broadly across different sample types. In comparison, a salt-mediated pH gradient entails the use of a compressed pH range along with a concurrent ionic strength gradient. With such an approach, it might be necessary to fine tune gradients and buffer concentrations, but the technique possesses the best capability for antibody charge heterogeneity.12,19,20
With these potential elution mechanisms in mind, we aimed to separately learn about ionization efficiency as a function of pH and ionic strength. To this point, Figure 2 shows the results of performing SEC-MS on NIST mAb with different ammonium acetate solutions, both varying in pH and concentration. In these data, a more dramatic decrease in ionization efficiency was observed when pH was changed from 5 to 7 to 9 versus when concentration was changed from 50 to 150 to 300 mM. Accordingly, the IonHance CX-MS mobile phases were designed around these data to provide an efficient salt-mediated pH gradient.
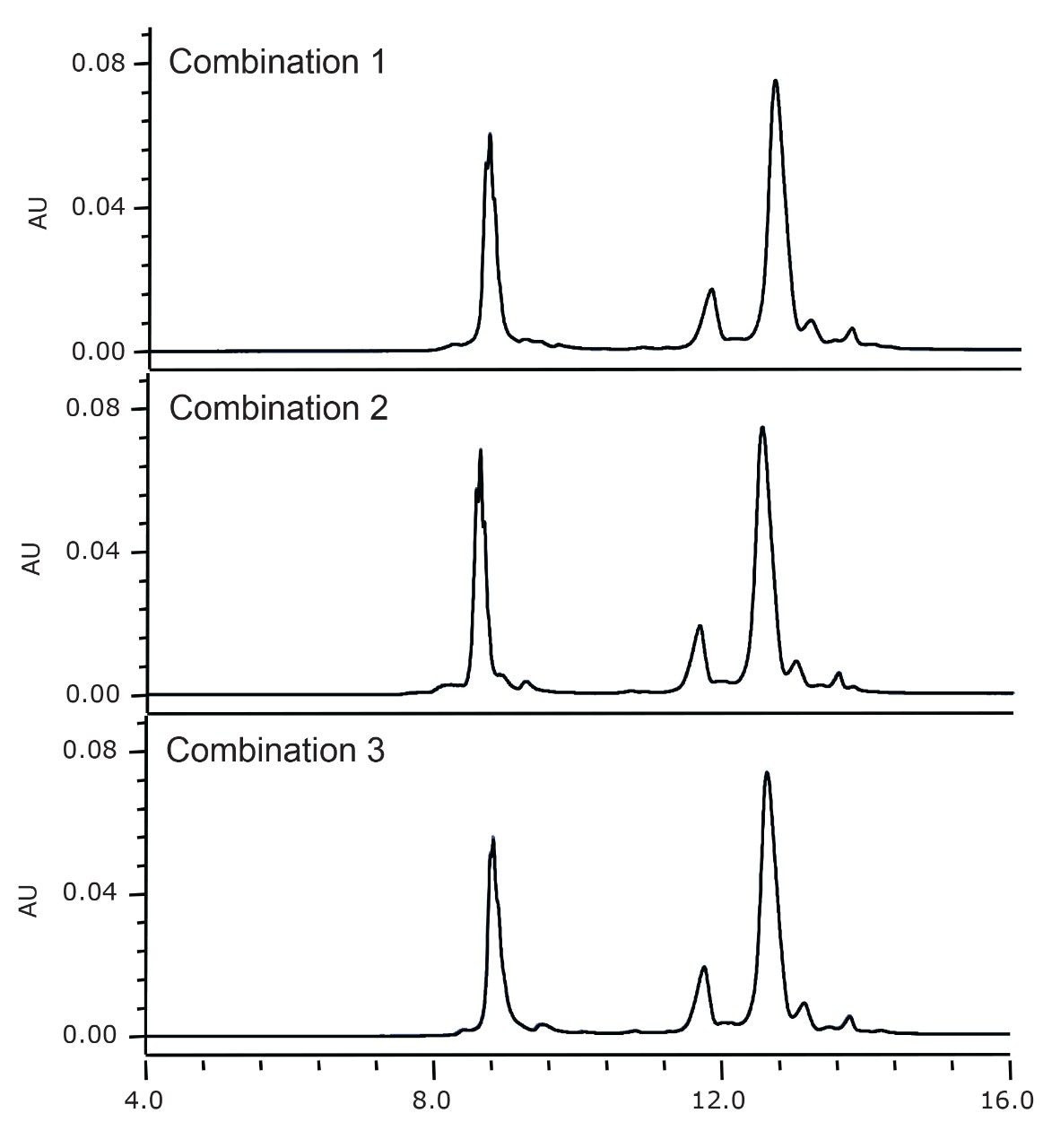
The IonHance CX-MS Concentrates have been formulated for a 10x dilution of mobile phase concentrates to deliver 50 mM pH 5.0 and 160 mM pH 8.5 ammonium acetate solutions which are capable of producing very robust separations, especially when combined with BioResolve SCX mAb Column technology. This robustness was evaluated through repeated separations of IdeS digested, non-reduced trastuzumab as performed with different batches of concentrates as well as different batches of column stationary phase. Chromatograms collected from this experiment are shown in Figure 3. As can be observed, the retention times of components in this separation were highly reproducible despite the use of different consumable batches. In fact, all components in the separation were found to possess incredible reproducibility, exhibiting retention times with RSD values <2%. Equally noteworthy is the resolution observed between the acidic variant and the main peak of the F(ab')2 subunit, which also showed an RSD <2%. In sum, a simple 10x dilution of the IonHance CX-MS pH Concentrates is a desirable and robust starting point for testing new analytes and establishing IEX-MS capabilities.
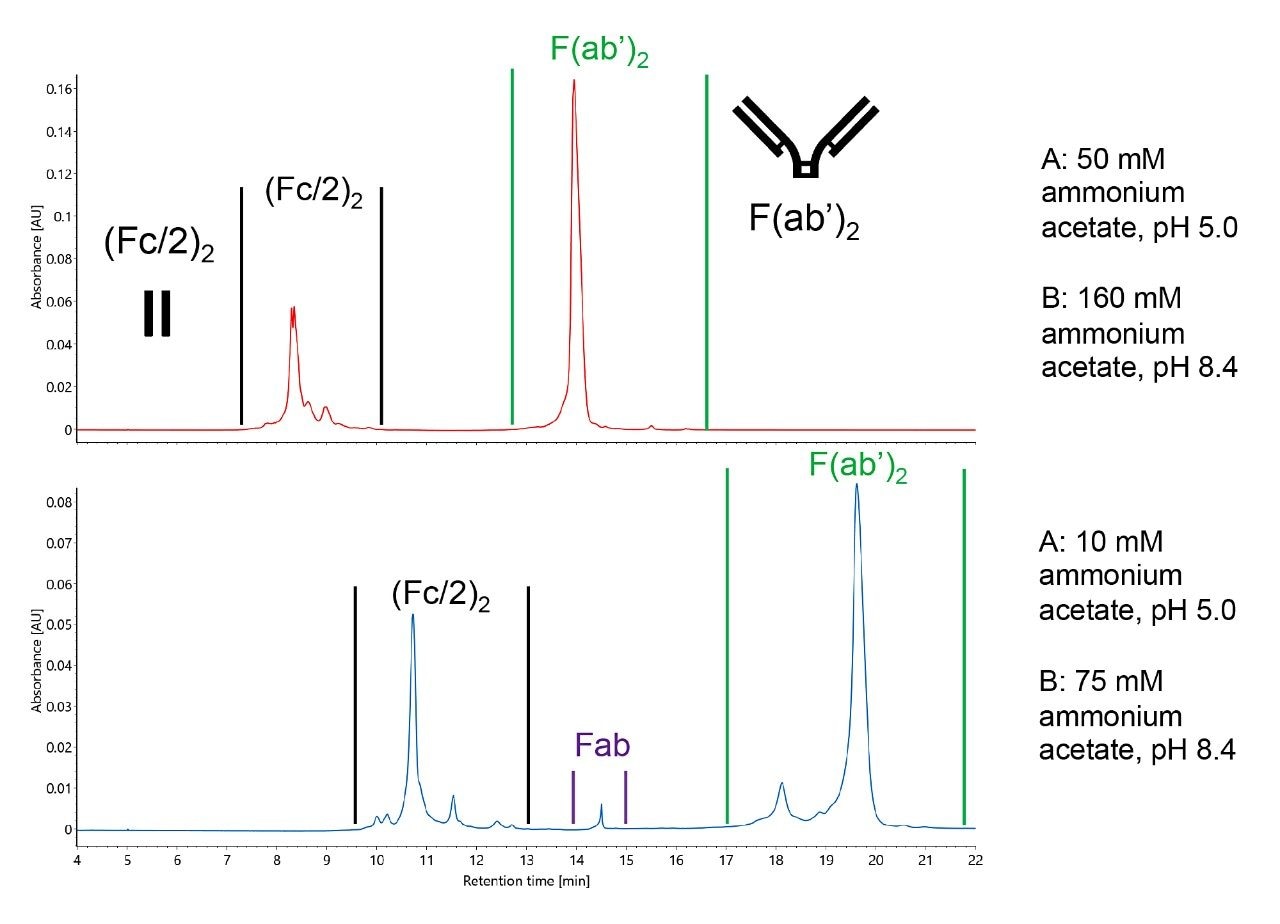
That said, it is unlikely for the standard dilution ratios to produce perfectly optimized separations for all analytes. Challenging samples might require tailored methods. For some samples, the ionic strength of the 10x diluted concentrates might cause undesirable analyte elution. Further dilution of the concentrates beyond 10x may improve such separations by enhancing the pH gradient mechanism and reducing ionic strength. Dilution of the CX-MS concentrates can alter pH, but the overall change is minor. For example, buffer A diluted to 10 mM ammonium acetate (50x dilution) remains at pH 5.0 whereas buffer B diluted to 75 mM ammonium acetate (21x dilution) has been measured to be pH 8.4. While there is potential to optimize separations with numerous different conditions, one should avoid conflicting separation conditions, specifically an ionic strength that decreases across a run. Dilution of both buffer solutions beyond the 10x dilution of the concentrates leads to delayed elution, provided the same gradient table is used, as evidenced in Figure 4 and two example separations of IdeS digested, non-reduced NIST mAb. Both the (Fc/2)2 and F(ab')2 subunit samples are shifted to longer retention times, yet the separation affords improved charge variant resolution.
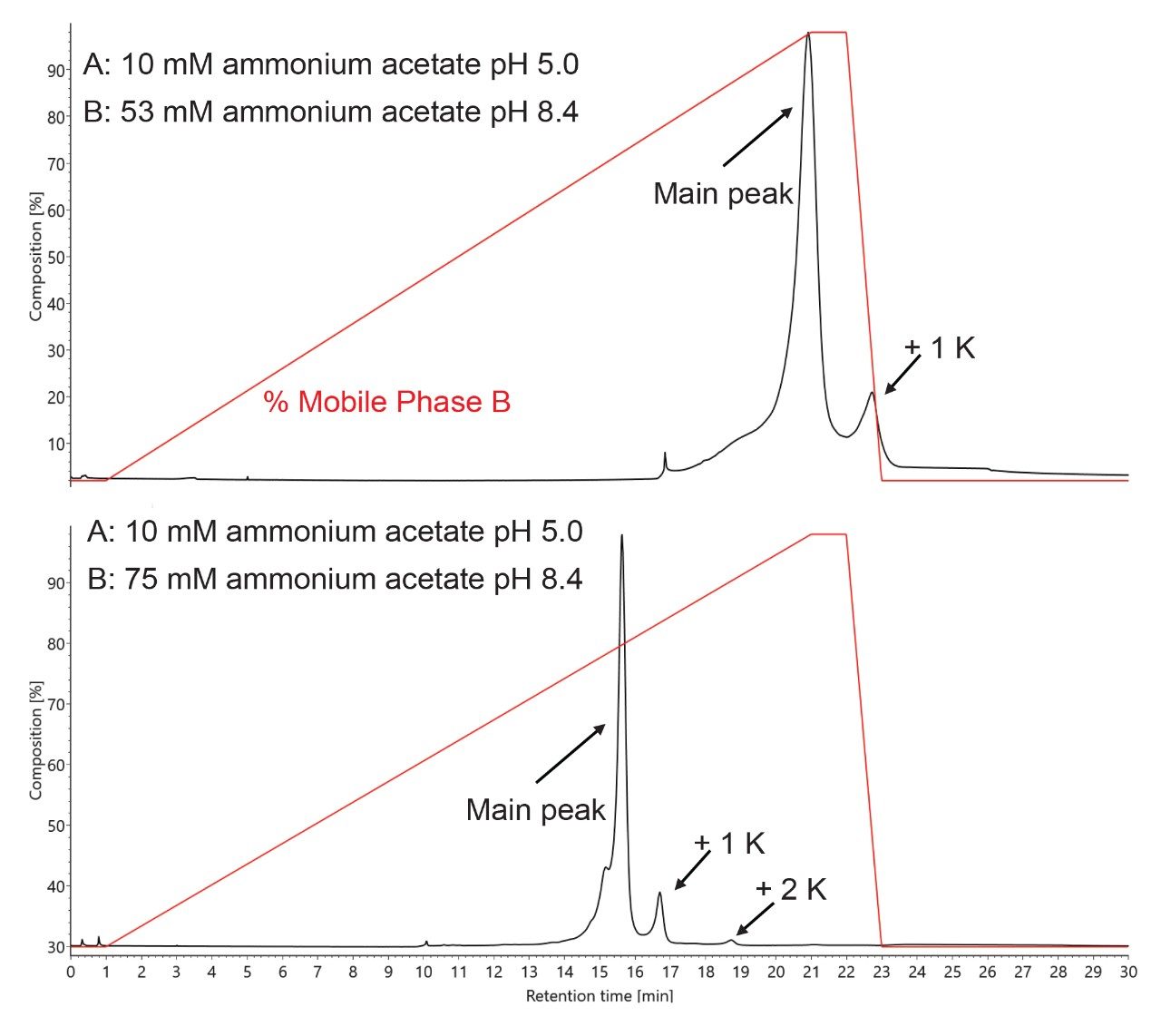
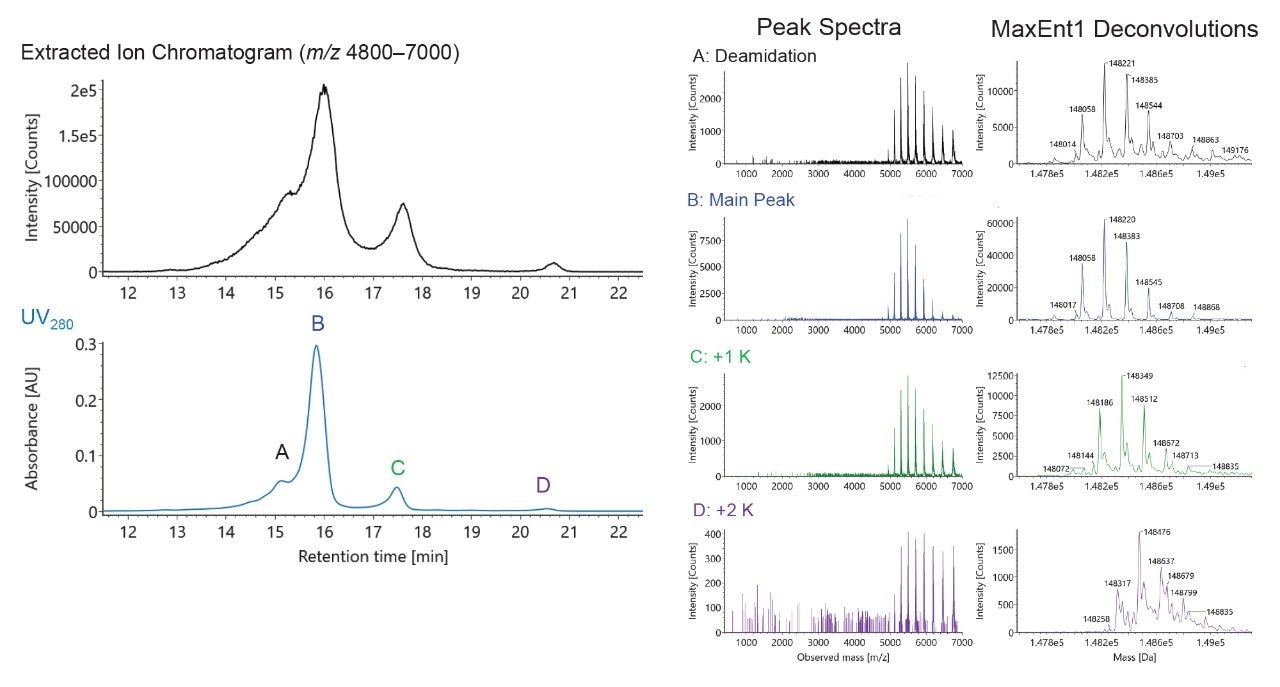
One looming concern is over dilution; Figure 5 presents a situation in which a high pI mAb, NIST mAb, was not fully eluted due to insufficient ionic strength (which came as a result of using 30x diluted pH Concentrate B, 53 mM ammonium acetate pH 8.4). In this instance, the +1 lysine (+1 K) variant eluted while the +2 K variant did not. To remedy this issue, an analyst can scout for the most suitable dilution. A more desirable separation is also shown in Figure 5, and it was tuned by switching to a concentration of 75 mM for mobile phase B (or 21x diluted CX-MS pH Concentrate B). With this separation and the purity of the mobile phases, it is possible to acquire high quality mass spectra. To this point, Figure 6 shows example raw mass spectra collected for intact NIST mAb, the corresponding deconvoluted mass spectra, and some example peak identifications, such as those for some low abundance variants.
Once a satisfactory ionic strength has been selected, the only remaining method parameter to optimize is the gradient, including the steepness in mobile phase change and the window of mobile phase compositions. With this, focused gradients can be developed for specific samples. Gradient optimization for IEX-MS is akin to other chromatographic techniques and can be used to shorten runtimes, control gradient steepness, and improved chromatographic resolution.
Charge variant analysis can be easily achieved through the coupling of ion-exchange chromatography with mass spectrometry, if appropriately formulated mobile phases are used. Literature has shown the advantages of salt-mediated pH gradients: they afford the ability to chromatographically focus and separate mAb charge variants. The IonHance CX-MS pH Concentrates provide mobile phases for robust separations and achieving high quality MS spectra. The availability of these concentrates should also allow analysts to quickly develop methods for analyzing both intact and subunit-digested samples. If it is of interest to optimize any given separation, two powerful tools exist for method development, namely alternative dilution factors and the fine tuning of LC gradients.
720006761, February 2020