
For research use only. Not for use in diagnostic procedures.
This application note illustrates the enhanced sensitivity obtained by using post column addition of isopropanol by analysis of ibuprofen metabolites in urine as an example.
The ionKey/MS System allows for high-sensitivity detection of polar metabolites with improved ionization process through post-column addition.
The importance of metabolite profiling of a new drug is well-recognized. Elucidation of drug metabolite information is crucial due to the fact that drug metabolites can be toxic at certain levels, have a greater pharmacodynamic effect than the parent drug, interfere with concomitant medication, and impact liver function. In drug discovery, liquid chromatography (LC) combined with electrospray ionization (ESI) mass spectrometry (MS) has become the method of choice for obtaining rapid metabolic information due to its ease of use, exceptional selectivity, and applicability, even toward thermally labile polar metabolites, such as glucuronides, sulfates, and glutathione conjugates.
However, in LC/ESI-MS the analyte sensitivity is dependent upon the nature of the analyte, as well as the mobile-phase properties, such as organic solvent and electrolyte contents.
It is accepted that a mobile phase with a higher composition of organic solvents improves the ionization efficiency, especially in the case of negative polarities.1 However, achieving the desired chromatographic separation of metabolites may require the use of mobile phases that may not be ideal for ESI. Altering solvent properties by post-column addition of a modifier can be an effective technique to improve sensitivity. Post-column addition of an organic modifier stabilizes the spray and allows enhancement of sensitivity without affecting the chromatographic separation.
An attractive aspect of the post-column addition is that the LC part is completely decoupled from the ionization region, which allows the use of an assortment of modifiers.
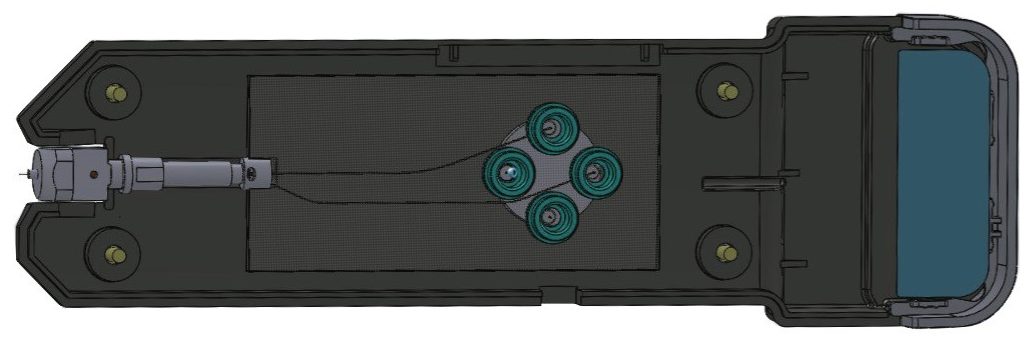
The post-column addition iKey (PCA iKey, Figure 1) used here is a microfluidic device containing two channels, one channel (the separation channel) is packed with sub-2-μm reversed-phase BEH C18 particles, and the other is an open channel used for post column addition of the solvent. The PCA channel joins the separation channel prior to the emitter. In this application, ibuprofen metabolites in urine was used as an example to illustrate the enhanced sensitivity obtained by using post-column addition of isopropanol.
Pre-dose urine and ibuprofen-metabolite-containing urine (3 hours after the oral administration of 200 mg) samples were collected from a healthy male volunteer. The urine samples were directly injected after a 1:50 dilution in water.
|
LC system: |
ACQUITY UPLC M-Class (Figure 2) |
|
Column: |
PCA iKey BEH C18 Separation Device, 130Å, 1.7 μm, 150 μm x 100 mm (Figure 1) |
|
Column temp.: |
65 °C |
|
Loop size: |
1 μL |
|
Injection volume: |
Full loop mode |
|
Flow rate: |
3.0 μL/min |
|
Mobile phase A: |
10 mM ammonium acetate buffer pH 4.5 |
|
Mobile phase B: |
Acetonitrile |
|
PCA solvent: |
Isopropanol |
|
Flow rate PCA: |
1.0 μL/min |
|
Weak needle wash: |
10 mM ammonium acetate buffer pH 4.5 |
|
Strong needle wash: |
50% acetonitrile, 25% methanol, 25% water |
|
Seal wash: |
90:10 water:acetonitrile |
|
Time (min) |
Flow (μL/min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
Initial |
3 |
95 |
5 |
initial |
|
10 |
3 |
35 |
65 |
6 |
|
11 |
3 |
5 |
95 |
6 |
|
14 |
3 |
5 |
95 |
6 |
|
15 |
3 |
95 |
5 |
6 |
|
MS system: |
Xevo G2-XS QTof (Figure 2) |
|
Ionization mode: |
ESI negative |
|
Capillary voltage: |
2.3 kV |
|
Source temp.: |
150 °C |
|
Cone voltage: |
50 V |
|
Source offset: |
150 V |
|
Quad profile: |
Auto |
|
Scan: |
50-600 Da, 0.1 second |
|
Data format: |
Centroid |
|
Analyzer mode: |
Sensitivity |
|
RF settings: |
Auto |

The analysis of human urine in negative ionization mode using post-column addition of isopropanol showed a significant increase in sensitivity (Figure 3). The separation was performed using a 3 μL/min gradient from 5 to 65% ACN in 10 minutes. Under standard gradient conditions, in the absence of isopropanol (Figure 3, top chromatogram), not all compounds were detected. Post-column addition of isopropanol allowed detection of more hydrophilic compounds, including ibuprofen metabolites. Additionally, adding isopropanol lowered the capillary voltage required for a stable electrospray, and therefore reduced the possibility of electric discharges that can generate undesired background noise.
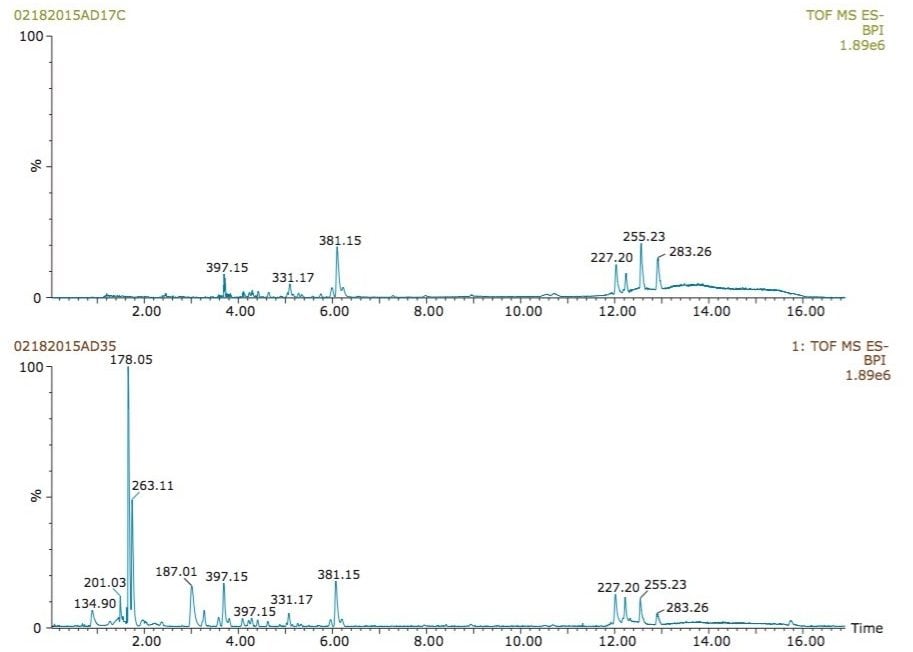
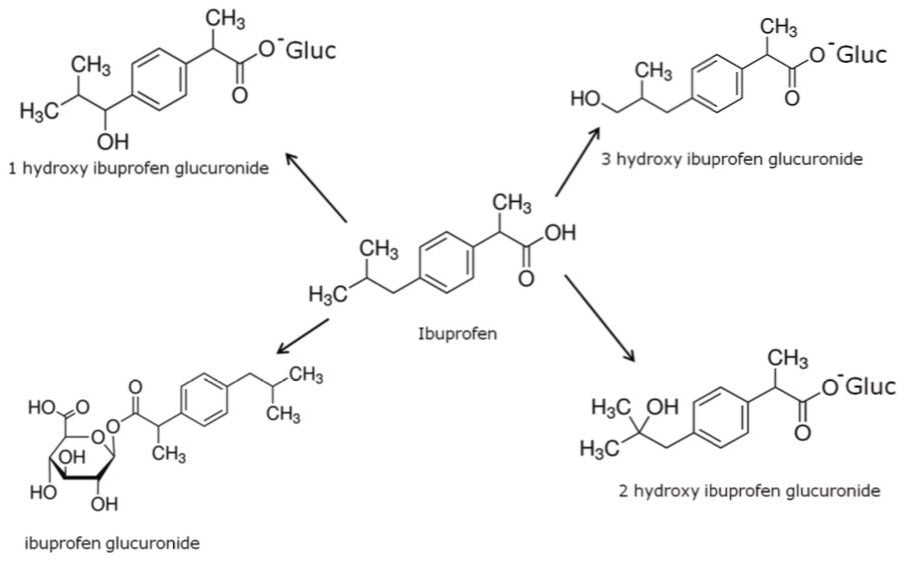
Figure 4 shows the chemical structure of ibuprofen and some of its major metabolites.
We examined the sample for evidence of ibuprofen metabolites using the combination of low (5 eV) and high (25 eV) collision energies to generate both molecular ion and fragment ion data.
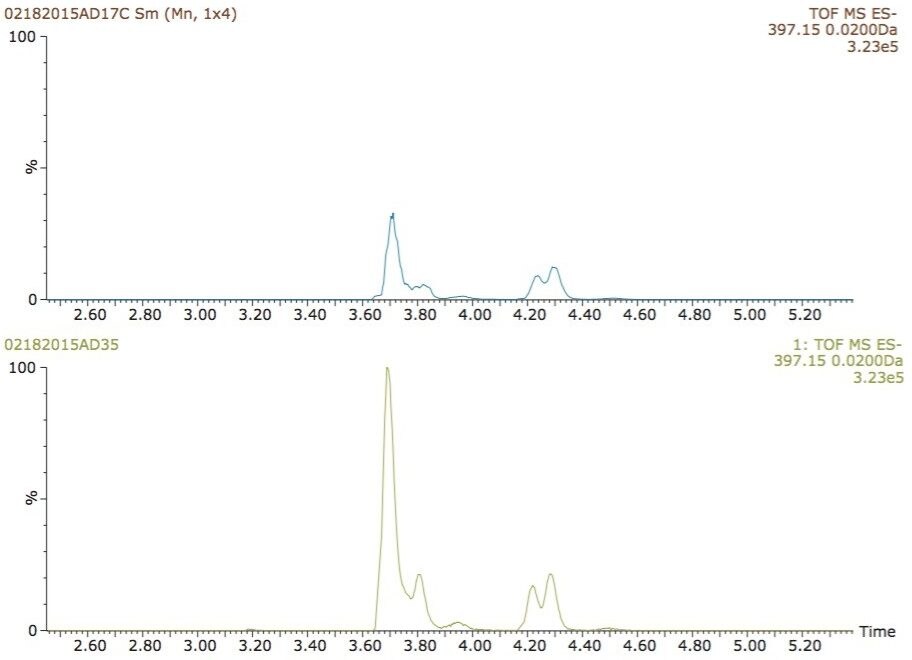
The extracted ion chromatograms for m/z 397.1499, corresponding to hydroxylated glucuronide metabolites of ibuprofen are shown in Figure 5. For the most intense peak eluting with a retention time of 3.7 min, the signal increased by over 50% when IPA was used (Figure 5, bottom chromatogram).
In the absence of the post-column addition of isopropanol, analytes that elute in low %ACN were mostly undetected or produced very low signals.
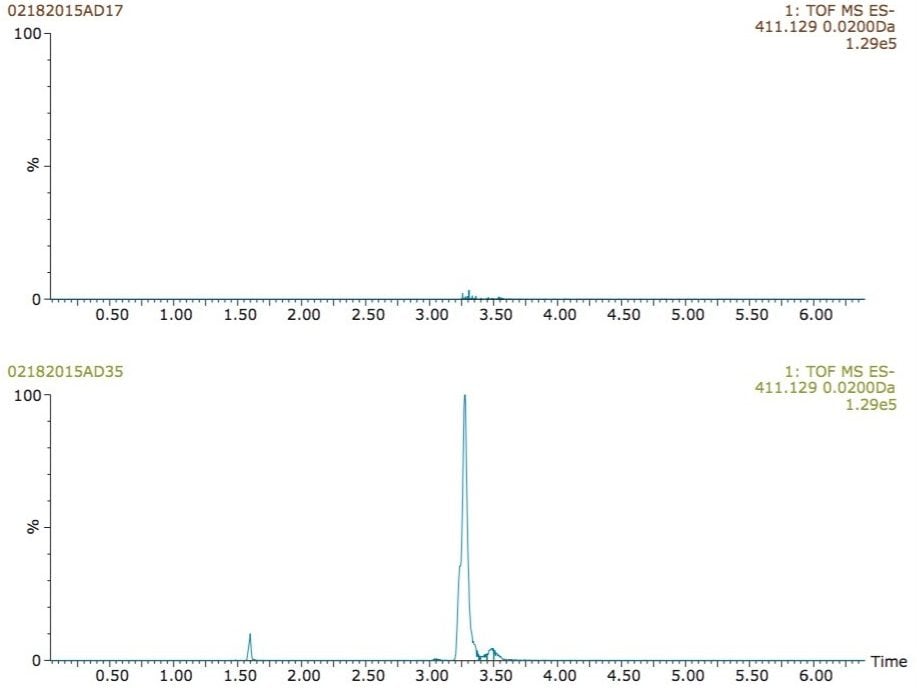
As illustrated in figure 6, another common ibuprofen metabolite with an m/z of 411.1279, formed by the further oxidation ot one of the side-chain-hydroxylated methyl group, was detected only when using post-column addition of isopropanol.
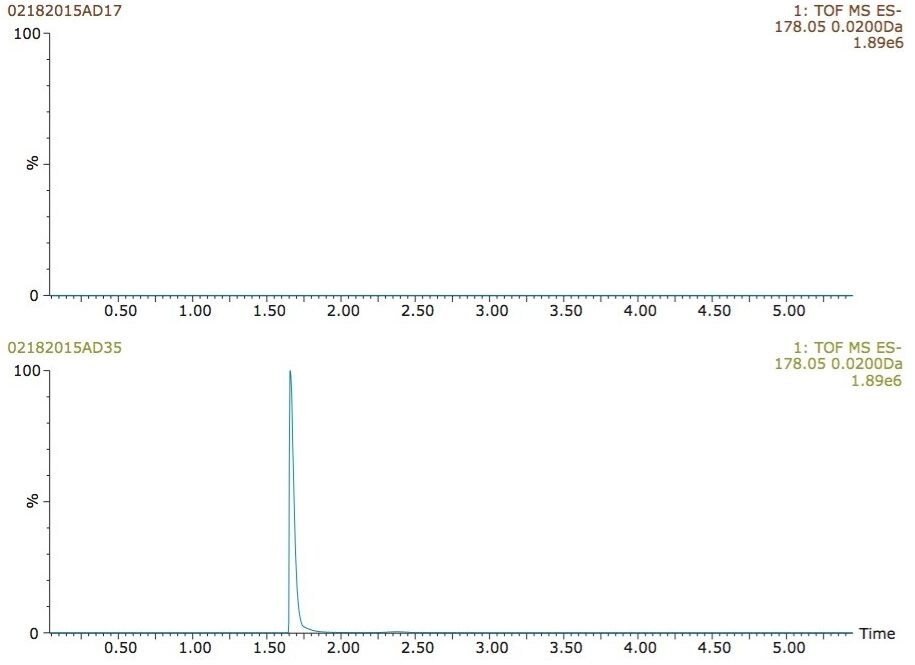
The advantage of this approach was further applied towards the detection of other urine metabolites. Hippuric acid (m/z=178.0504), an endogenous polar biomarker produced an intense signal in the presence of isopropanol, but was completely undetected under standard conditions (Figure 7).
In low-flow negative-ion mode electrospray ionization, post-column addition of an organic solvent stabilized the electrospray and demonstrated improved sensitivity. Ibuprofen and associated metabolites, as well as hippuric acid, proved the utility of post-column addition to generate high-quality metabolic profiling data.
While this study is demonstrating the advantage of post-column addition in negative ionization mode, we foresee other applications where adding a post-column reagent can enhance the results.
720005365, May 2015