
For research use only. Not for use in diagnostic procedures.
In this application note, we demonstrate the application of UPC2 for the analysis of polar analytes in aqueous matrices.
UltraPerformance Convergence Chromatography (UPC2) can be used for the direct analysis of polar metabolites from aqueous biological samples, such as urine, or from extracted serum samples. Analytical methods can be rapidly developed from a simple set of starting conditions of column flow-rate, temperature, and pressure. Modifying the co-solvent by the addition of small amounts of water buffer and acid provides rapid high resolution analysis with rapid re-equilibration times.
Liquid chromatography (LC) coupled with accurate mass, mass-spectrometry has become the technique of choice for the screening of biological fluid such as urine and plasma for small molecule biomarkers. While reversed-phase LC can successfully analyze moderately polar analyst, lipids, and non-polar steroidal metabolites, the analysis of highly polar analytes still represents a challenge. The ability to analyze these biological samples with minimal pre-treatment is critical as the introduction of multiple sample preparation steps can result in a reduction in analytical precision and/or loss of analyte species due to poor solvent solubility. Hydrophilic interaction liquid chromatography (HILIC) has shown some success for the analysis of highly polar compounds, however the long re-equilibration times and low tolerance to highly aqueous sample loading limits its utility. Here we explore the application of UPC2 for the analysis of polar analytes in aqueous matrices.
Caffeine was employed as a model polar compound in order to investigate the choice of injection parameters for the analysis of polar compounds by UPC,2 as it maintains a constant charge across a wide pH range (pKa = 14.2) and is both PDA and ES+ active. A standard solution was prepared at a concentration of 0.1 mg/mL-1 in 9:1 heptane:isopropanol. To investigate the feasibility of aqueous sample injection using UPC,2 0.1 mg/mL-1 solutions of caffeine were prepared using water, methanol (MeOH), ethanol (EtOH), isopropanol (IPA), acetonitrile (ACN), acetone (Me2CO), and 9:1 heptane:IPA as solvents. Water miscible organic solvents were chosen in order to appraise their potential as diluents for aqueous solutions during the same set of experiments. Each working solution was analyzed on the ACQUITY UPC2 System with PDA Detector, with injection volumes ranging from 1–7 μL.
The chromatography was performed on an ACQUITY UPC2 System, comprising of an ACQUITY UPC2 Binary Solvent Manager, ACQUITY UPC2 Sample Manager, Automatic Back-Pressure Regulator (ABPR), and an ACQUITY Photo Diode Array (PDA) Detector. The separation was performed on ACQUITY UPC2 BEH Column (1.7 μm, 3.0 x 100 mm) and eluted with an isocratic mobile phase comprising of 95:5 (v/v) CO2:MeOH, operated at 2.5 mL/min.-1 The density of the subcritical fluid was regulated via a column temperature of 55 °C and automatic backpressure regulator (ABPR) pressure setting of 2,000 psi. The column effluent was monitored by PDA at 273 nm.
|
LC system: |
ACQUITY UPC2 |
|
Detection: |
UV 273 nm |
|
Column: |
ACQUITY UPC2 BEH Column (1.7 μm, 3.0 x 100 mm) |
|
Column temp.: |
55 °C |
|
Sample temp.: |
Room temperature |
|
Injection volume: |
1 μL/min |
|
Flow rate: |
2.5 mL/min-1 |
|
Mobile phase A: |
CO2 |
|
Mobile phase B: |
Methanol isocratic mobile phase comprising of 95:5 (v/v) CO2:MeOH |
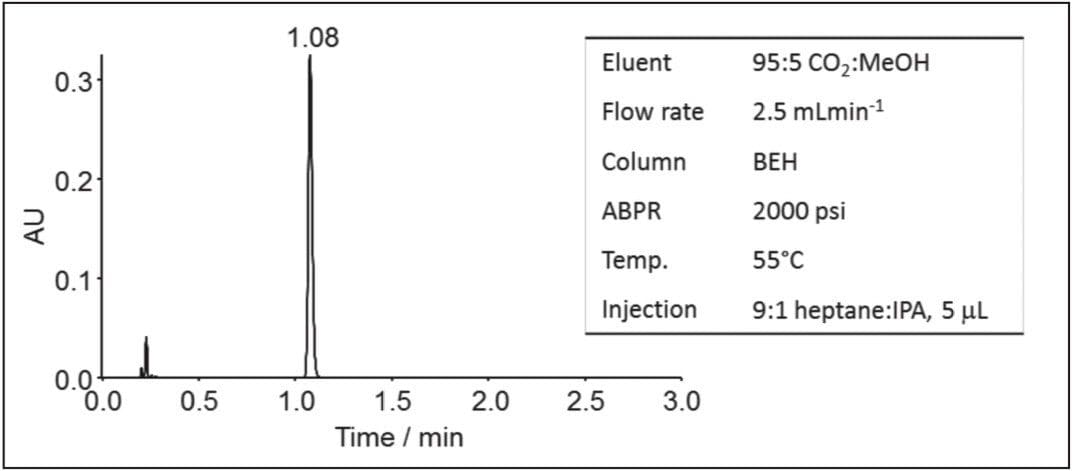
The ACQUITY UPC2 System produced a highly efficient peak with a mean N value of 18,910 +/- 193 being recorded. An example chromatogram is given in Figure 1. This illustrates that both the ACQUITY UPC2 System and chromatography column were performing correctly.
Very few studies have managed to successfully introduce analytes for SFC as solutions in water without severe disruption to chromatographic peak shapes.1-3 The effect of different injection solvents was investigated over the injection range of 1-7μL. The polarity ranged from the very polar water and methanol through to the aprotic solvent acetone to the very non-polar heptane/IPA mix. The effect of the injection solvent was evaluated by comparing the efficiency of the observed peak to that of the 9:1 heptane:isopropanol injection solvent and expressed as Nrel, where a value >1 represents an improvement in performance and a value <1 represents a reduction in chromatographic performance. The data obtained is presented below in Figure 2.
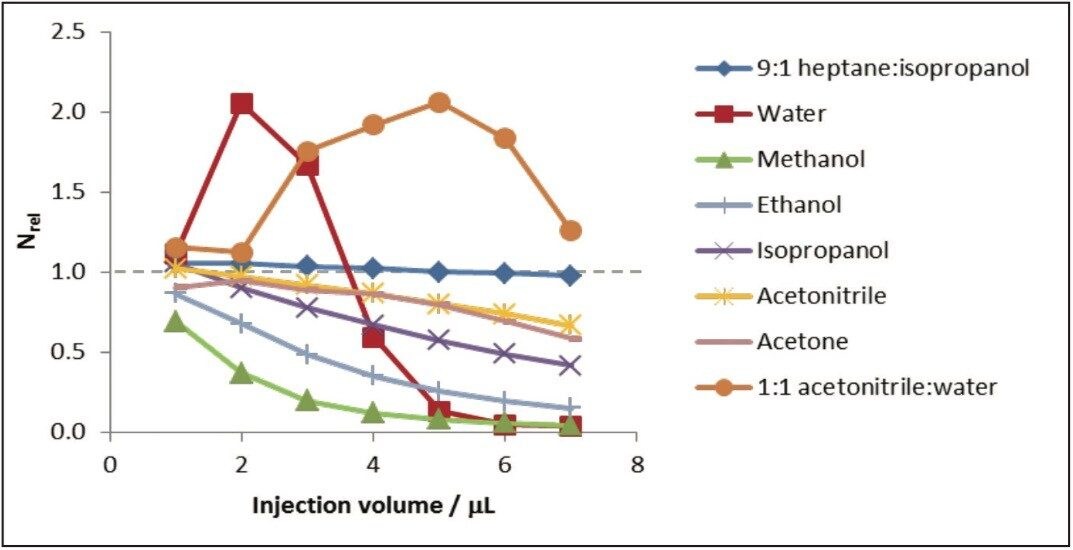
Heptane:IPA gave the smallest changes in Nrel with changing injection volume (1.06-0.98), and this is due to the close similarity in polarity to the effluent mobile phase. The water miscible organic solvents gave the poorest efficiencies, progressively deteriorating as solvent polarity increased (i.e. in general, Nrel ACN > Me2CO > IPA > EtOH > MeOH). The sequence presumably reflects the increasingly poor mismatch in polarity to the eluent. The organic solvents generally showed an anticipated steady decrease in Nrel from 1 to 7μL injection as a result of the increasing size of the injection plug. Of the water miscible solvents, acetonitrile and acetone showed the smallest changes in Nrel (1.02–0.67 and 0.91–0.58, respectively), and methanol the greatest change (0.70–0.04).
Water behaved somewhat unexpectedly as an injection solvent. The maximum efficiency (Nrel = 2.05) was significantly higher than for heptane:IPA, despite the poorer mismatch in polarity to the eluent. This maximum efficiency occurred at 2 μL as opposed to 1 μL injection volume, which was the observed optimal injection volume for all of the other pure solvents. The efficiency Nrel quickly reduced with injection volumes greater than 2 μL, with peak splitting observed in aqueous injection volumes of 5 μL or greater. Interestingly the use of an acetonitrile:water as the injection solvent sowed and increase in Nrel increased up to nearly 6 μL. Although the use of stronger elution injection solvents resulted in marginally earlier elution than heptane:IPA, reductions in retention times were less than 10%. Accordingly, injection in water was not only tolerated in UPC,2 but effected a reduction in peak broadening at some injection volumes. Of the water miscible organic solvents, acetonitrile showed greatest promise as a potential diluent for aqueous solutions.
Several factors could account for the beneficial effect of water on the UPC2 efficiency for the analysis of caffeine, including improved mass transfer with the polar BEH phase or focusing (reduced longitudinal diffusion) of the elution band. To better assess the influence of water on the chromatographic system as a whole, further investigations were made via modification of both the injection solvent and the co-solvent with water.
To assess the chromatographic benefits of water as an injection solvent irrespective of how it was introduced into the ACQUITY UPC2 System, a 0.1 mg/mL-1 caffeine solution in the various solvents was analyzed by the ACQUITY UPC2 System with PDA Detector, using a modification to the method in which 95:5 MeOH:H2O was used as the co-solvent instead of pure methanol. The derived data is shown below in Figure 3. The results show that for injection volumes of less than 2 μL, the observed efficiencies for water injections were higher than those obtained for all of the organic injection solvents.
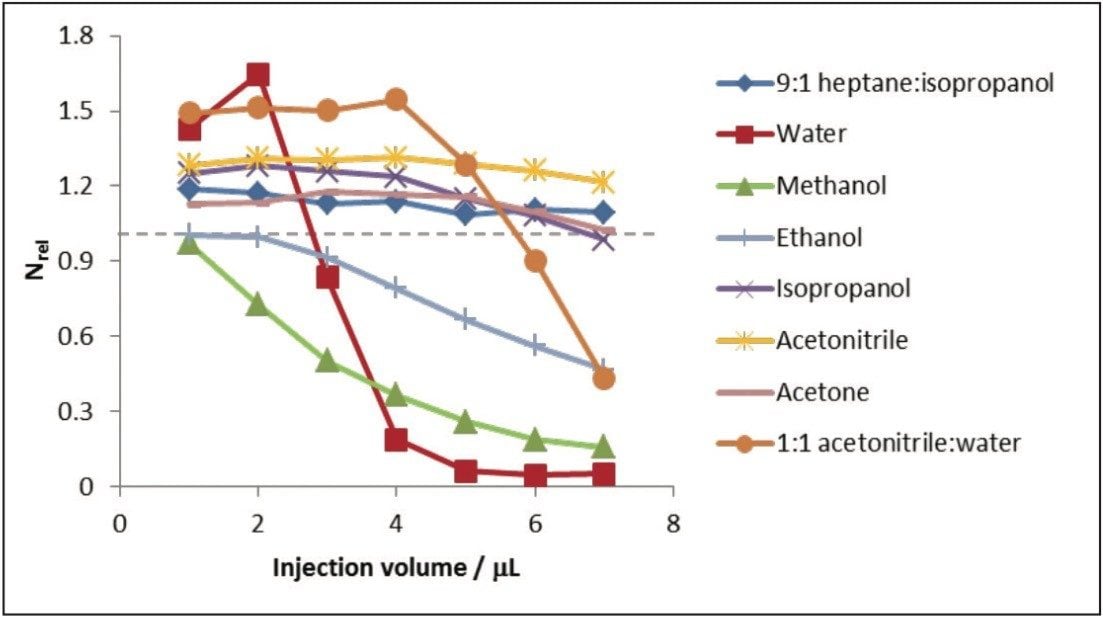
Interestingly, acetonitrile replaced heptane:IPA as the most tolerant injection solvent, giving the smallest changes in Nrel with increasing injection volume (1.37–1.27). Injection in water still yielded the optimal efficiencies, although with smaller magnitudes (maximum Nrel =1.72), but these were shifted towards lower injection volumes compared to the non-water containing co-solvent data. The results suggest that water is inherently beneficial to the chromatographic analysis of caffeine whether introduced via the co-solvent or injection solvent.
The reduction in UPC2 efficiency observed in this study at higher aqueous injection volumes suggests a limit to the amount of water that can be injected without deleteriously affecting the chromatography. To determine the dependence of chromatographic performance on the volumetric injection of aqueous solutions, a 0.1 mg/mL-1 solution of caffeine was prepared in 1:1 acetonitrile:water and analyzed using an ACQUITY UPC2 System with PDA Detector, either with MeOH or 95:5 MeOH:H2O as co-solvents. In each case, the maximum efficiency was similar to that obtained via injection of pure water but occurred at approximately double the injection volume, suggesting the volume of the aqueous component was the critical factor, not the total injection volume.
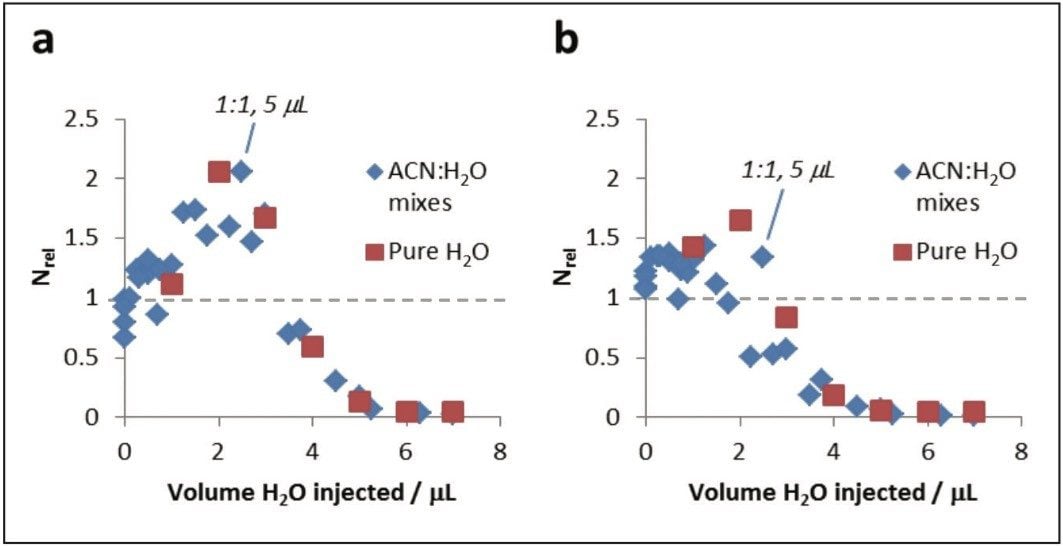
In order to identify the maximum amount of water tolerated by UPC,2 caffeine solutions comprising 0, 10, 33, 50, 67, 90, and 100% water were made by diluting a 1 mg/mL-1 aqueous caffeine stock with acetonitrile to give a total volume of 1 mL. Each solution was then analyzed by using an ACQUITY UPC2 System with PDA Detecor, using methanol or 95:5 methanol : water as the co-solvent, with the injection volume varied from 1–7 μL.
Plots of Nrel against the volume of water injected are given in Figure 4. The data shows that a maximum in chromatographic performance occurs when the injection volume contains approximately 2 μL of water. It can be seen that injections with greater volumes of water produced significantly poorer efficiencies irrespective of the co-solvents. By contrast, injection of lesser volumes of water were highly detrimental to the chromatographic efficiency in MeOH co-solvent, but only moderately detrimental with the MeOH:H2O (95:5) co-solvent. The results suggest that the total volume of water injected should be kept ≤2 μL for UPC,2 irrespective of the composition of the injection solvent.
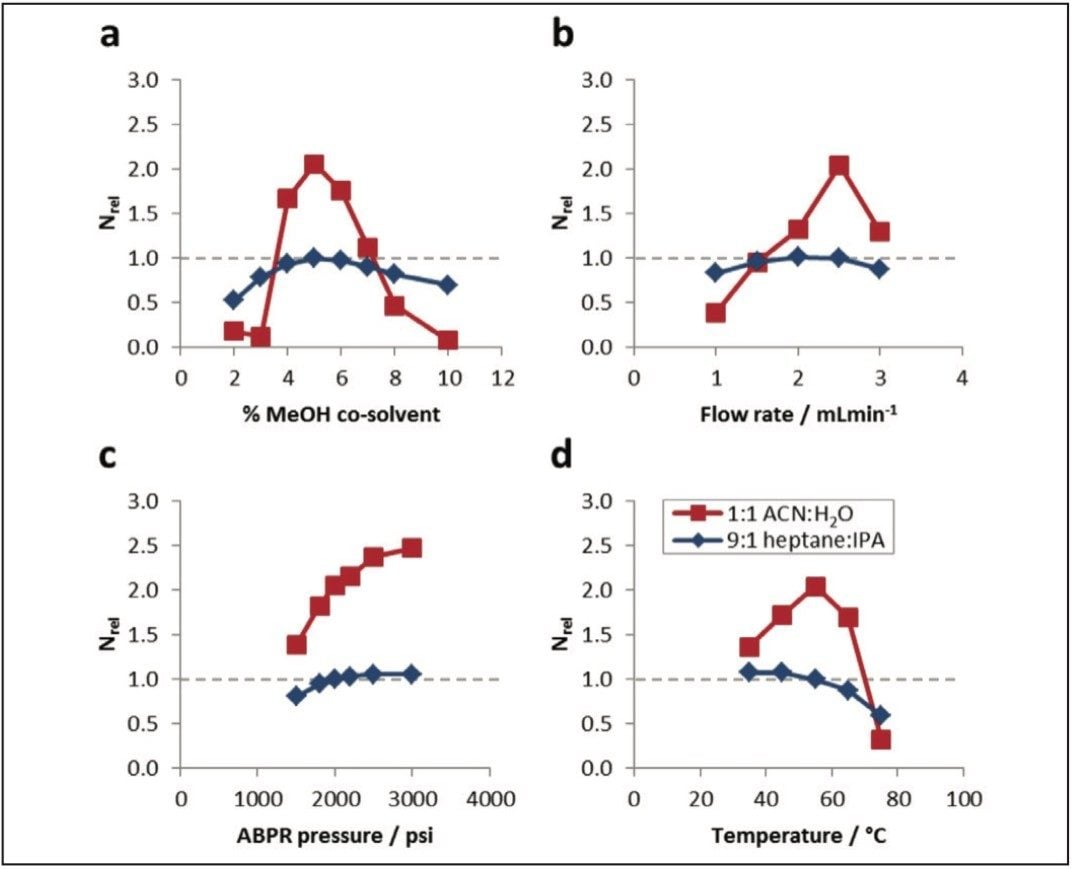
Along with the injection conditions, changes to the ACQUITY UPC2 System operating parameters could also enhance, diminish or destroy the beneficial effects of water on the chromatographic performance of UPC2 for polar analytes. To determine the optimal operating parameters and robustness to changes in these conditions the proportion of co-solvent in the eluent (2–10% methanol), the eluent flow rate (1.0–3.0 mL/min-1), the ABPR pressure (1,500–3,000 psi), and the column temperature (35–75 °C) were independently varied in order to screen for any influences on system efficiency (Nrel). For these experiments, 1:1 acetronitrile:water was employed as the injection solvent and a 5 μL injection volume was chosen as previous data suggested that these conditions gave optimal or near-optimal efficiencies with either MeOH or MeOH:H2O co-solvents. A 5 μL injection of caffeine in 9:1 heptane:IPA was employed to determine Nrel.
From a careful interrogation of the data shown in Figure 5 it can be determined that the parameters which gave optimal efficiencies were largely the same for both aqueous and control solutions, with the notable exception of those for column temperature, which optimized at 55 °C and 35 °C, respectively. For both injection solvents, Nrel values increased with increasing ABPR pressure up to 3000 psi. For each of the system parameters, Nrel was far more sensitive to systematic change when the aqueous injection solvent was employed rather than heptane:IPA (9:1) This data suggest that particular care should be taken to optimize the parameters when using aqueous solutions, as small changes can severely diminish the chromatographic efficiency.
720005505, September 2015