For research use only. Not for use in diagnostic procedures.
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates that the use of an online SPE system (ACQUITY Online Sample Manager (OSM)) enables simultaneous and analytically sensitive measurement of a panel of androgens.
Online SPE has a dramatic impact on the measurement of androgens in serum
The measurement of male androgens in most clinical research laboratories is limited to only measuring testosterone. To more accurately determine androgen status in men, the measurement of other androgens such as dihydrotestosterone (DHT) and dehydroepiandrosterone (DHEA), would be useful. However, measuring other androgens can be difficult without derivatization and chromatographic separation. We report here a combined LC-MS method for the measurement of testosterone (T), androstenendione (A4), DHT, and DHEA on a small sample volume of serum.
Zinc sulfate (100 μL, 50 g/L) was added to 100 μL of sample. After mixing, acetonitrile (100 μL) containing internal standards (D2 T, D7 A4, D2 DHEA, and D3 DHT) was added and mixed for 1 minute. The samples were then centrifuged at 1,700 g for 10 minutes before analysis.
Sample (75 μL) was extracted using an online automated solid phase extraction using a C18 SPE cartridge. Cartridges were initially conditioned with methanol and equilibrated with water.
Calibrators were made in phosphate buffered saline containing 0.1% (w/v) bovine serum albumin. The measuring range was up to 50 nmol/L for T, A4, DHEA, and up to 5 nmol/L for DHT.
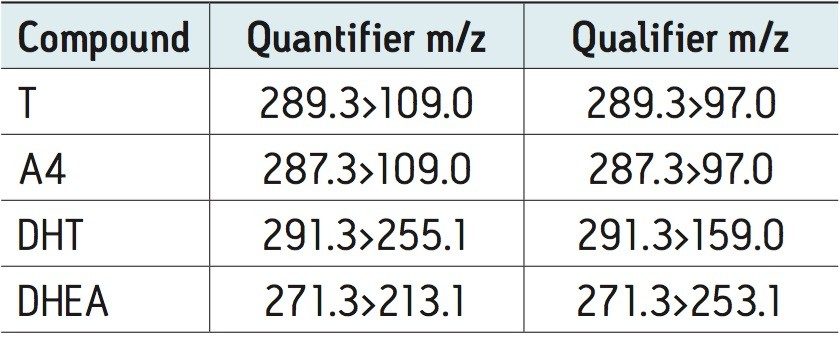
Eluent was directed (without stream splitting) into the ion source of a Waters Xevo TQ-S tandem quadrupole mass spectrometer operated in the positive ion mode using the following quantifier and qualifier transitions:
A clinical research method for simultaneously measuring four androgens (T, A4, DHT, and DHEA) from serum has been developed. This method takes advantage of the unique capabilities of an online SPE system (ACQUITY OSM) to enable analytically sensitive and reproducible measurements of these steroid hormones.
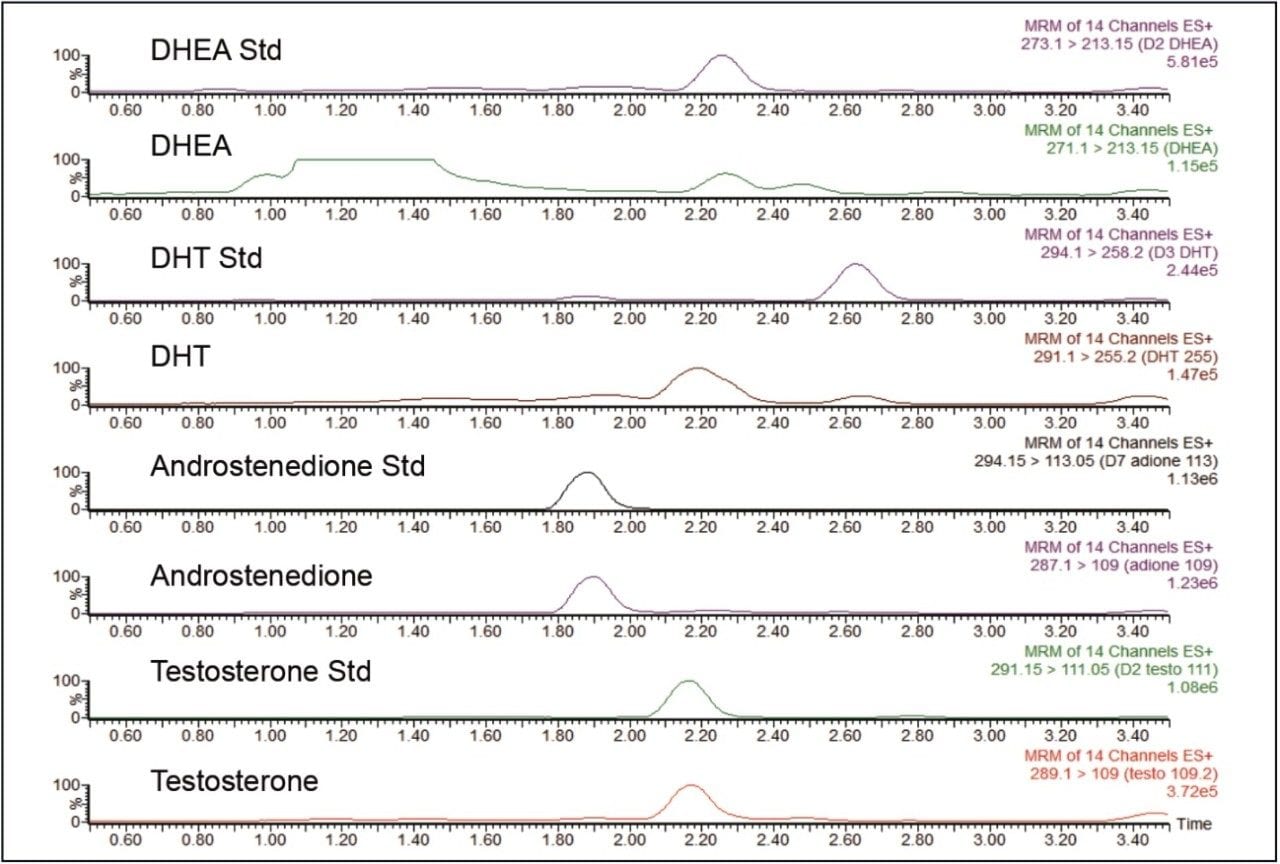
Chromatographic separation was achieved between all four androgens (see Figure 2 below). The run time was 6.5 minutes per sample. Negligible ion suppression was observed using a post-column infusion of the internal standards when extracted male and female samples (3 of each) were injected.
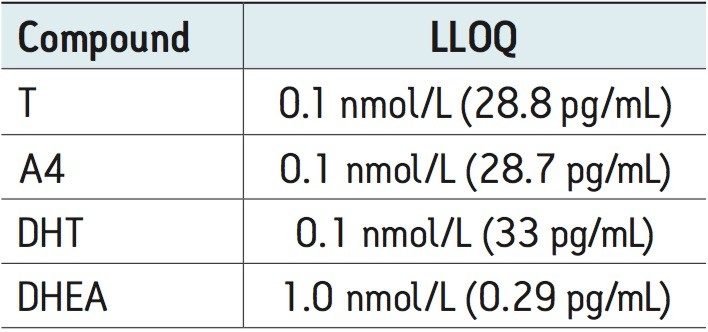
Calibration lines were linear (r2 >0.99) to at least 50 nmol/L for T, A4, DHEA, and 5 nmol/L for DHT. Table 2 shows the lower limit of quantitation for each of the androgens in both male and female samples.
The average recovery range (n=6) for each compound was; T 98% (86 – 103%), A4 101% (83 – 107%), DHEA 94% (82 – 112%), and DHT 91% (83 – 107%).
When compared with an existing single analyte LC-MS method for measurement of these androgens, testosterone and androstenedione gave the following comparisions: Testosterone (combined) = 1.01 x existing method + 0.07 nmol/L and androstenedione (combined) = 1.09 x existing method - 0.29 nmol/L.
The development of a rapid clinical research method for the LC-MS/MS measurement of testosterone, androstenedione, DHT, and DHEA in a clinical research laboratory is described here. The method requires a very small volume of sample (100 μL), and all four androgen analytes are measured simultaneously without a lengthy and complex derivatization procedure. In addition, separation of the four androgens was achieved using a relatively short (50 mm) column that was also able to separate any potential isobaric interferences (e.g. D2 T and DHT and DHEAS in DHEA) as shown in Figure 2.
This clinical research method is fast and relatively simple to implement, and has the potential to analyze large numbers of samples measuring multiple androgens in a single injection. These results were achieved by using a combination of efficient online SPE sample preparation in conjunction with a high analytical sensitivity mass spectrometer.
The method developed here provides:
720005051, August 2014