For forensic toxicology use only.
This application note highlights the analysis of a comprehensive panel of opiates, benzodiazepines, and other drugs of abuse. Using either Waters’ 2.7 μm CORTECS C18 Column, or a 2.5 μm XBridge BEH Phenyl XP Column, all compounds were analyzed within 4 minutes with excellent peak shape and narrow peak widths. Whether laboratories prefer the performance and efficiency of the solidcore/superficially porous CORTECS C18 Column, or the unique selectivity of the XBridge BEH Phenyl XP Column, each can be used to rapidly analyze this important group of compounds.
In forensic toxicology, drug screening panels often include such commonly used substances such as opiates, benzodiazepines and stimulants. These panels are often analyzed by LC-MS using traditional C18 column technologies. Key considerations include the ability to chromatographically resolve the various pairs of isobaric compounds included in these panels, while maintaining good peak shape for a variety of compounds. In addition, when using traditional HPLC systems, the ability to analyze samples as rapidly as possible without exceeding the pressure limitations of the system is very important. This application note highlights the capabilities of Waters’ new CORTECS C18 2.7 μm Columns and XBridge BEH Phenyl XP 2.5 μm Columns for this type of application. In the case of the CORTECS C18 Column, the high efficiency packing of solid core 2.7 μm particles yields excellent performance that equals or exceeds competitive columns at lower operating backpressures. If alternative selectivity is desired, the phenyl functionality of the BEH phenyl column enhances the retention of opiate compounds. This enhanced retention can potentially result in reducing ion suppression from urinary matrix components. Both columns achieve excellent baseline separation between isomers, and the entire panel of 35 compounds, including opioids, benzodiazepines, stimulants, and other drugs of abuse can be analyzed in under 4 minutes at backpressures compatible with any HPLC system.
Stock solutions were obtained from Cerilliant Corporation, Round Rock, TX. Stock solutions were prepared in methanol. Working solutions were prepared in 5% acetonitrile containing 0.1% formic acid.
|
LC system: |
ACQUITY UPLC I-Class, Fixed Loop (FL) with Column Manager (CMA) |
|
Columns: |
CORTECS C18 2.7 μm, 3.0 x 50 mm (p/n 186007370) XBridge BEH Phenyl XP 2.5 μm, 3.0 x 50 mm (p/n 186006069) |
|
Column temp.: |
30 ˚C |
|
Sample temp.: |
10 ˚C |
|
Injection volume: |
10 μL |
|
Mobile phase A: |
MilliQ water with 0.1% formic acid |
|
Mobile phase B: |
Acetonitrile with 0.1% formic acid |
|
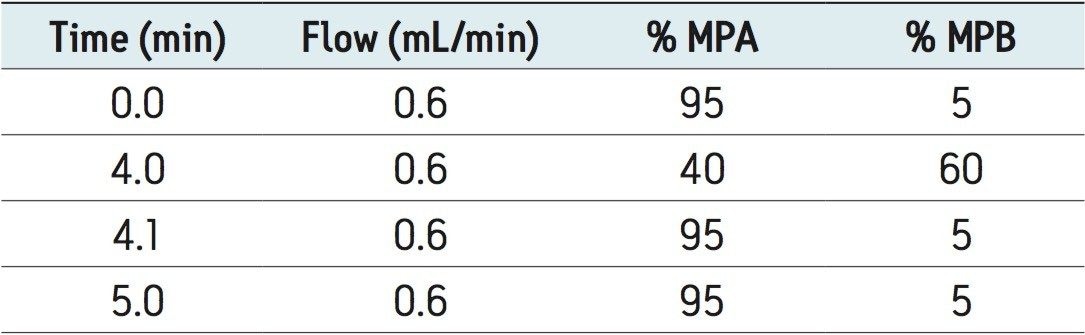
The mobile phase gradient is listed in Table 1. |
|
MS system: |
Xevo TQD |
|
Ionization mode: |
ESI Positive |
|
Capillary voltage: |
0.5 V |
|
Collision energy: |
Optimized for individual components |
|
Cone voltage: |
Optimized for individual components |
|
Data management: |
MassLynx v 4.1 scn 855 Software |
Waters CORTECS C18 2.7 μm Column, an XBridge BEH Phenyl XP 2.5 μm Column, and a competitor’s biphenyl core shell column (2.6 μm) were used to analyze a panel of 35 common pain management compounds (Figure 1), including opioids, benzodiazepines, stimulants, benzoylecgonine (BZE), and phencyclidine (PCP). All columns had the same dimensions (3.0 x 50 mm). The solvent gradient is listed in Table 1. The entire gradient cycle was 5 minutes.
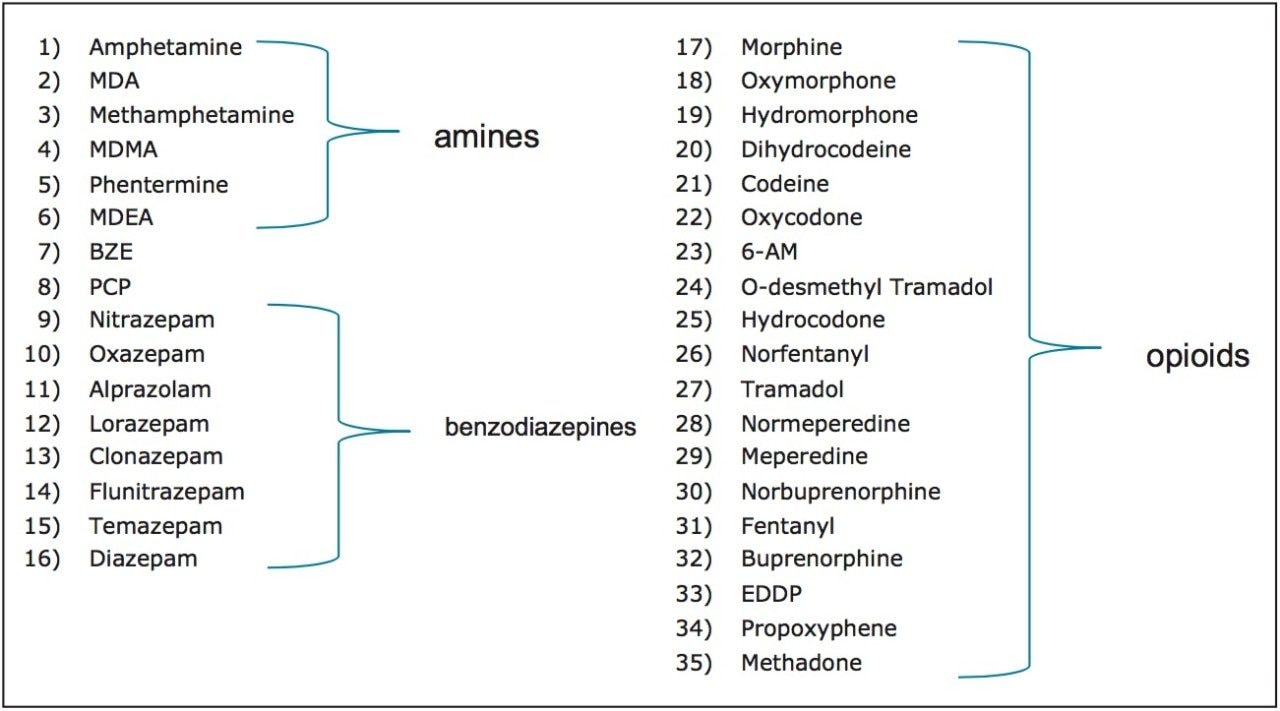
All compounds eluted within 4 minutes and showed good, symmetrical peak shape. Average peak width and maximum backpressure for all columns are shown in Table 2.
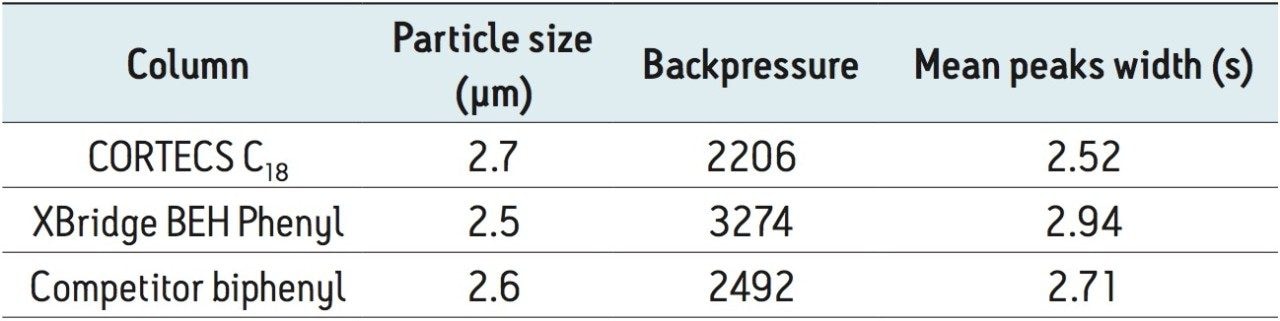
The columns operated at backpressures well within the limit of traditional HPLC systems and, predictably, backpressure increased with decreasing particle size. Interestingly, the CORTECS C18 Column, despite its larger particle size, demonstrated the best resolution, as measured by average peak width (see Table 2). The chromatography of all opioid compounds is shown in Figure 2a and the separation of key isobaric opiates can be seen in Figure 2b. All opioid drugs elute within 3.5 minutes and demonstrate good peak shape. As Figure 2b shows, the isobaric pairs of morphine and hydromorphone (peaks 17 and 19), and codeine and hydrocodone (peaks 21 and 25) are well separated on all columns. This is an important feature as these compounds must be resolved from each other for accurate identification and quantification. While the BEH phenyl and biphenyl column both show increased retention of these compounds, which is most likely a result of their phenyl functionality, excellent resolution is easily achieved on the CORTECS C18 Column.
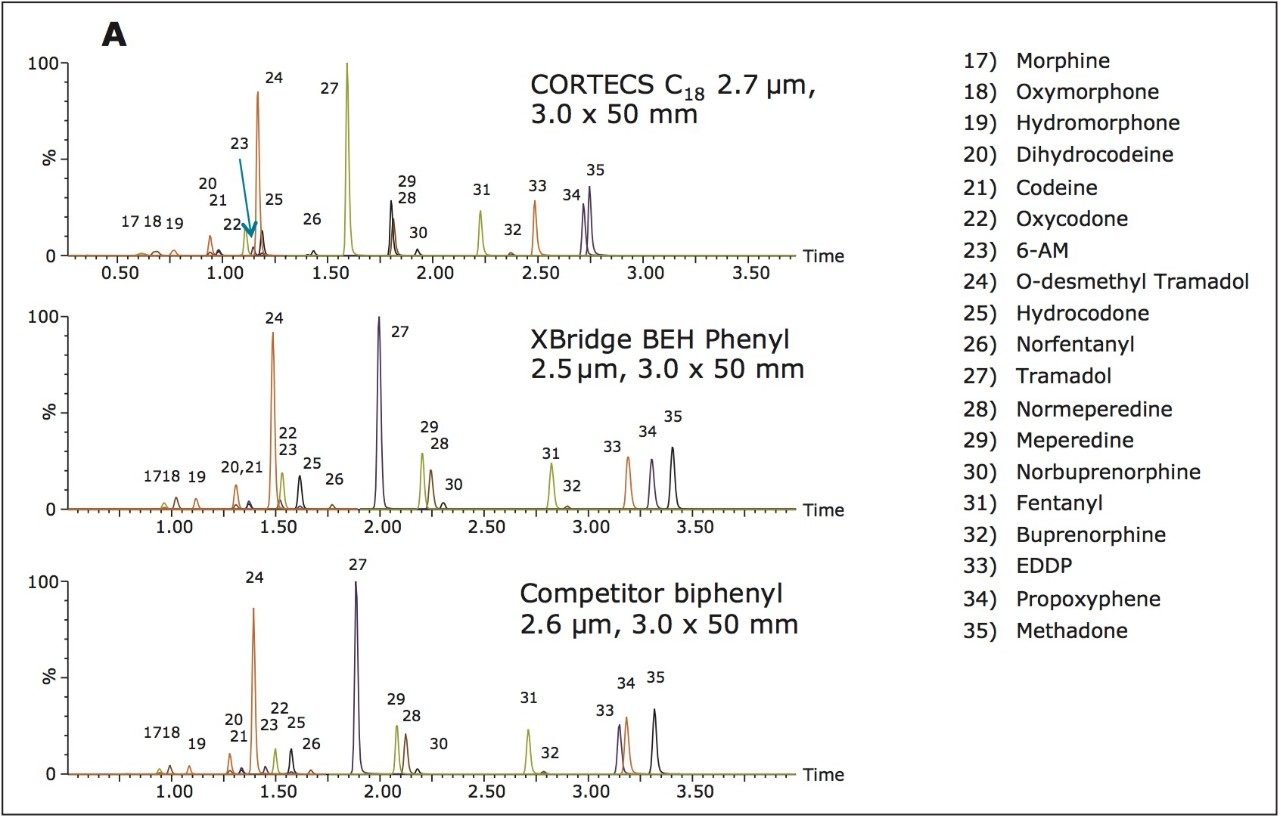
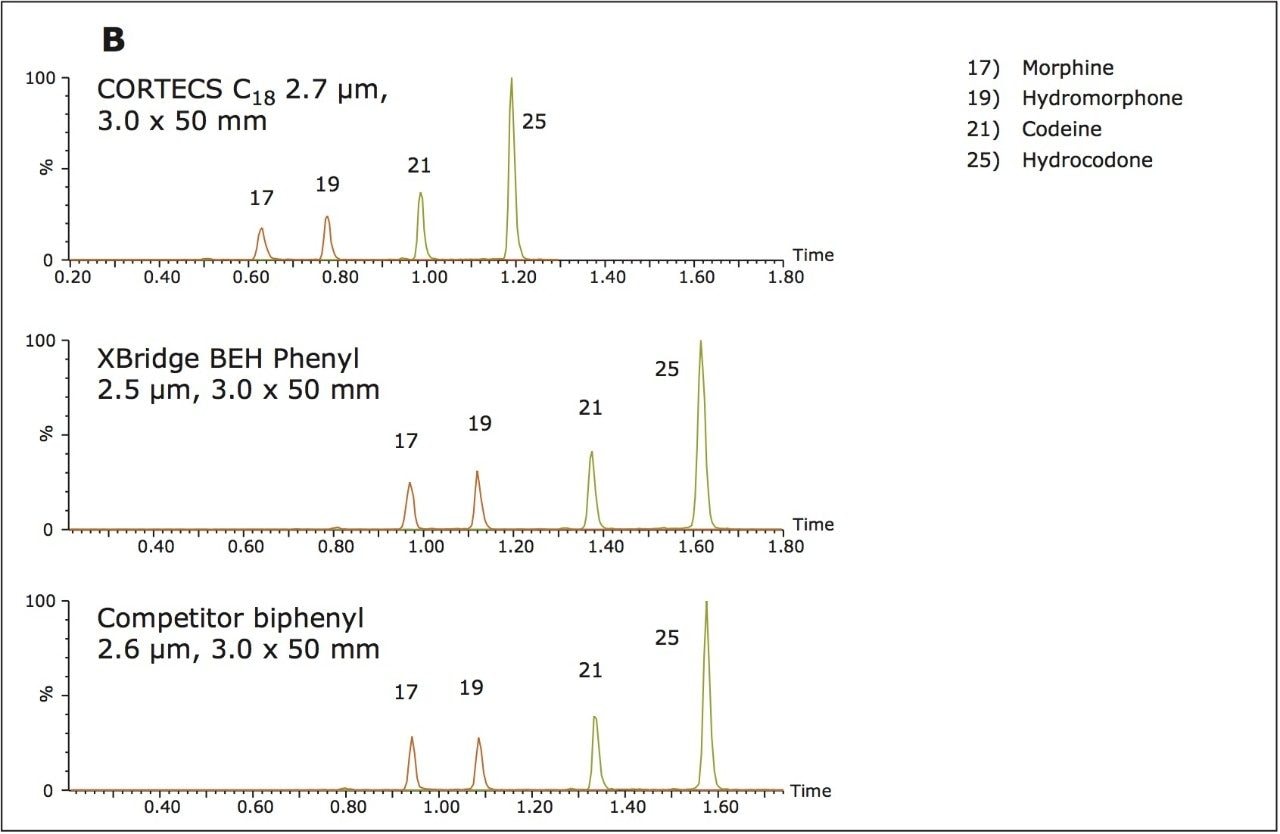
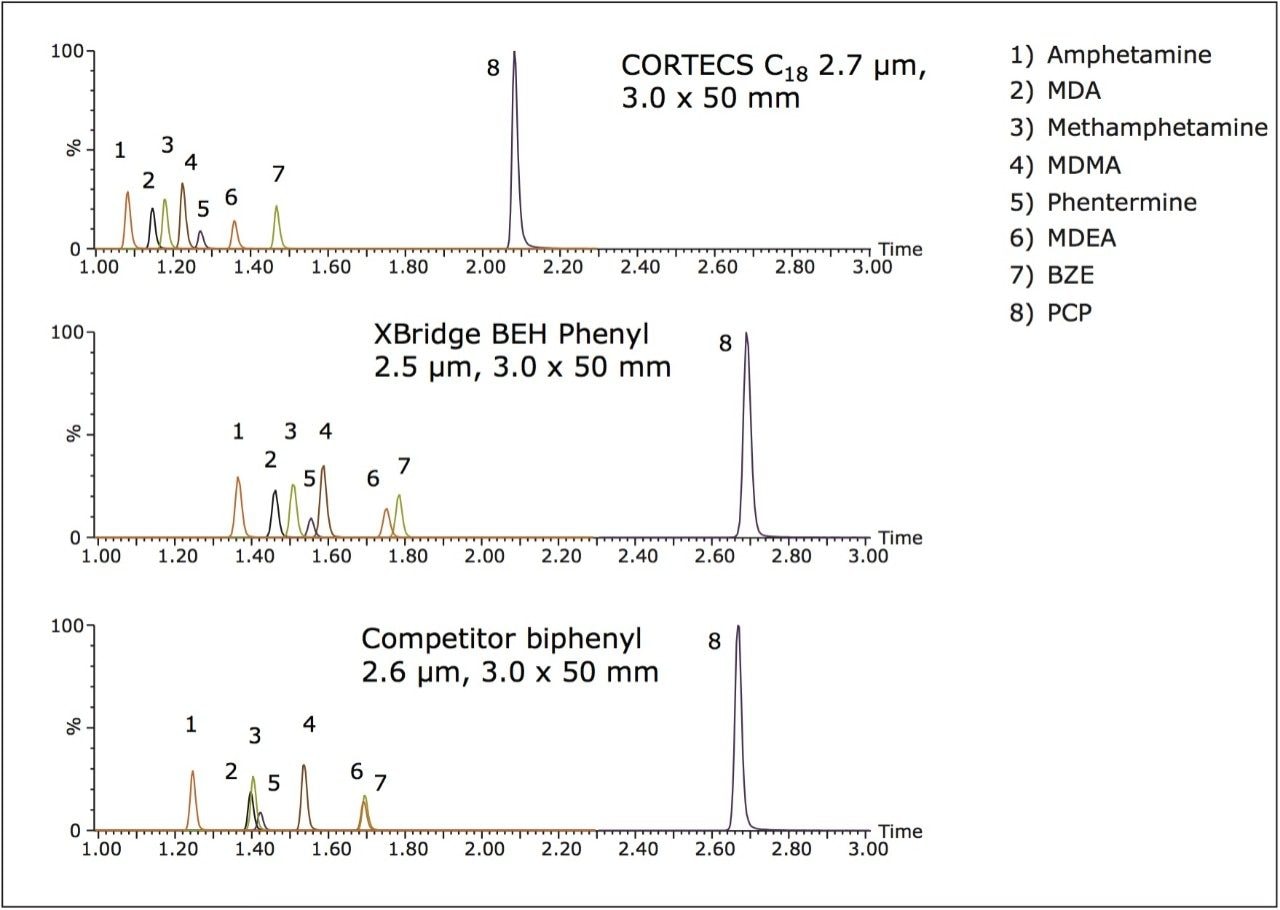
Figure 3 shows the chromatography of the amines, PCP, and BZE. While all peaks demonstrate good peak shape, the CORTECS C18 Column and XBridge BEH Phenyl XP Column both show excellent separation of these compounds. Of particular note are methamphetamine and phentermine (peaks 3 and 5) which demonstrate baseline separation on these two columns, but co-elute on the biphenyl column. This is an important feature as these compounds have identical molecular formulas and both have a major fragment ion at m/z 91. The ability to separate these compounds eliminates the risk of cross talk between these two stimulants and can be crucial to unambiguous identification. Figure 3 also demonstrates that MDEA and benzoylecgonine (peaks 6 and 7), which coelute on the biphenyl column, are separated on both the C18 and BEH phenyl columns.
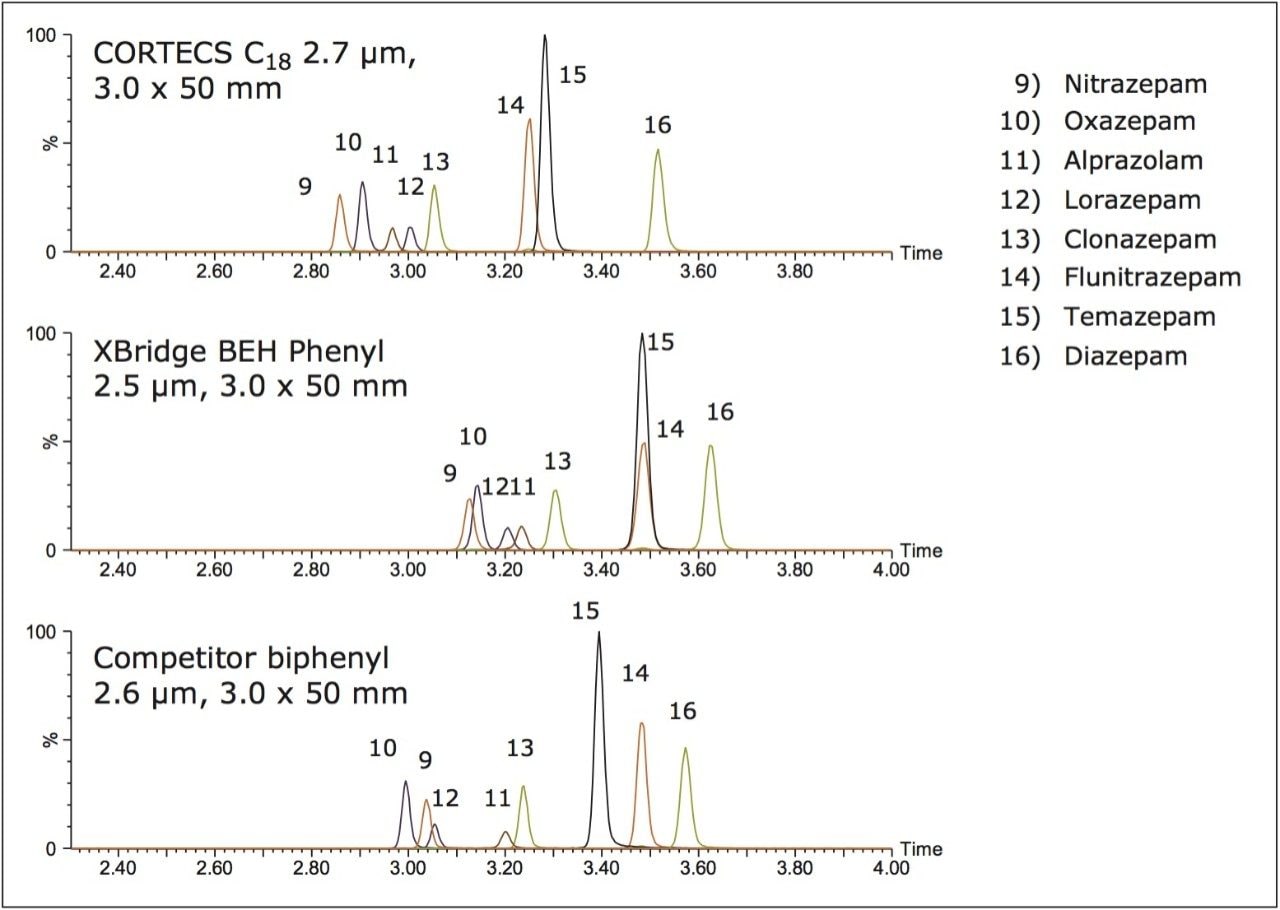
The benzodiazepine chromatography is shown in Figure 4. Good peak shape can be achieved on all columns. Once again, the CORTECS C18 Column, despite its larger particle size, demonstrates the highest resolution for this group of compounds (average peak width of 2.89 s).
This application note highlights the analysis of a comprehensive panel of opiates, benzodiazepines, and other drugs of abuse. Using either Waters’ 2.7 μm CORTECS C18 Column, or a 2.5 μm XBridge BEH Phenyl XP Column, all compounds were analyzed within 4 minutes with excellent peak shape and narrow peak widths. Maximum backpressures were were respectively 2206 and 3274 psi, enabling the use of these columns on traditional HPLC systems. Perhaps most importantly, baseline separation was achieved between isobaric compounds, allowing for their unambiguous identification and quantification. Whether laboratories prefer the performance and efficiency of the solidcore/superficially porous CORTECS C18 Column, or the unique selectivity of the XBridge BEH Phenyl XP Column, each can be used to rapidly analyze this important group of compounds.
720005185, September 2014