This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrate successful performance of the USP method for mometasone furoate ointment using a robust and reliable instrument with a repeatable solvent delivery designed for analytical QC methods.
The Alliance HPLC System delivered results with excellent reproducibility, essential to minimize downtime and enhance laboratory throughput for routine analyses.
Mometasone furoate ointment is a prescription skin medication that belongs to the group of synthetic corticosteroids used to relieve inflammation and itching caused by various skin problems, such as dermatitis, psoriasis, and eczema. It is applied directly on the affected areas of the skin to reduce inflammation and suppress an overactive immune system.
Compendial methods adopted into the laboratory for batch testing must be verified using a reliable and robust instrument. All compendial methods are deemed to be verified if the system suitability requirements defined in the individual USP monographs are met. Successful verification of the method is essential for laboratories to remain in compliance with the current Good Laboratory Practices regulations (cGMP).
Maintaining reproducible retention times is an important function of an LC system. These can be achieved with a high level of performance provided by precise solvent delivery, injection-to-injection accuracy, and consistent temperature control. Fulfilling the system suitability criteria of the USP monograph for routine analysis minimizes downtime while increasing analytical throughput.
Solutions used in this study were prepared according to the assay method defined in the USP monograph for mometasone furoate ointment. The USP method was run accordingly on an Alliance HPLC System equipped with a 2489 UV/Visible Detector and an appropriately selected Waters column guided by the Waters Reversed-Phase Selectivity Chart. The USP designates an L60 packing for the assay testing. Using the Waters Selectivity Chart, an XBridge Shield RP18 4.6 x 250 mm, 5 µm Column was chosen. Performance of the method was verified by evaluating the system suitability parameters based on the acceptance criteria defined in the USP monograph. The system suitability results for the mometasone furoate ointment method on the Alliance HPLC System passed the USP requirements, as summarized in Table 1. All values were substantially lower than the USP criteria. The Alliance HPLC System delivered results with excellent reproducibility, essential to minimize downtime and enhance laboratory throughput for routine analyses.
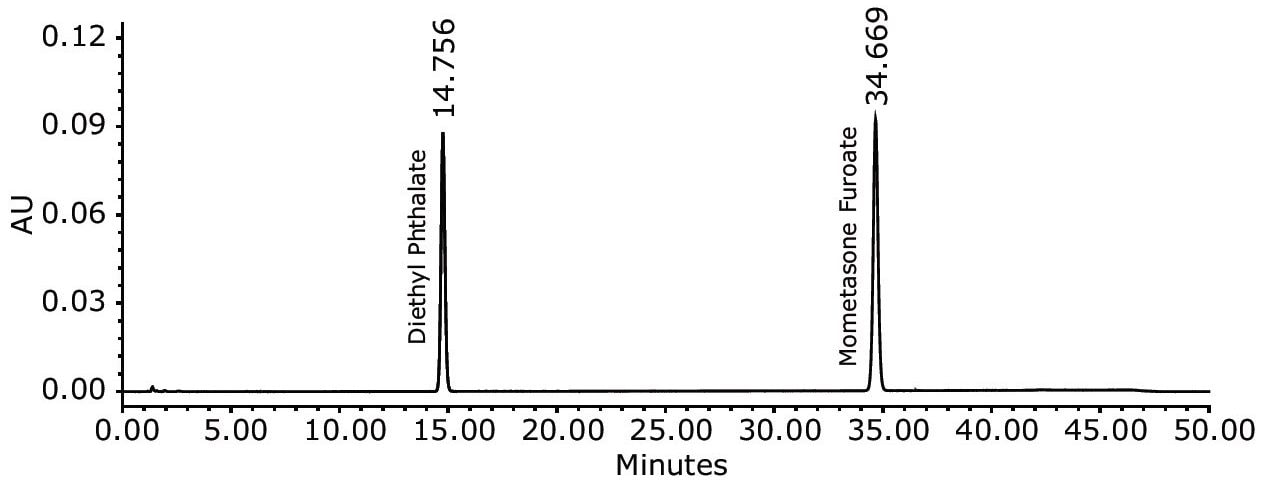
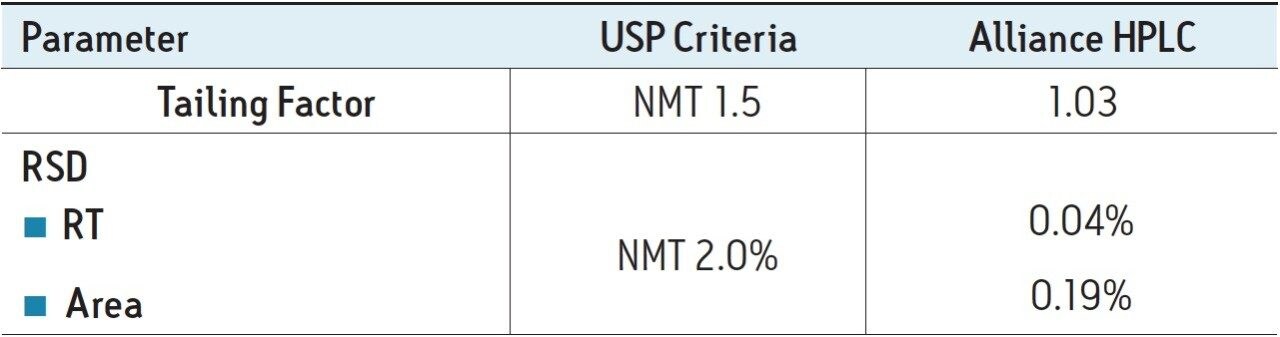
Excellent performance of the USP method for mometasone furoate ointment was achieved with the Alliance HPLC System. The assay method was effectively verified by meeting the USP requirements for system suitability parameters. The results of successful method verification demonstrated that the Alliance HPLC System is a reliable and robust instrument that meets the expectations of today’s laboratory to generate analyses with excellent precision and reproducibility, of key importance to eliminate downtime and improve laboratory efficiency.
720004540, January 2013