This is an Application Brief and does not contain a detailed Experimental section.
This application brief compares the ionization and fragmentation characteristics of pesticides using traditional electron ionization (EI+) GC-MS and APGC to improve MRM analysis of these compounds.
Coupled with the Xevo TQ MS or Xevo TQ-S, APGC enables low-level quantification of persistent organic pollutants that have proved challenging to analyze using EI+ GC-MS systems.
The Stockholm Convention (2004) is an international treaty that aims to eliminate or restrict the production and use of certain persistent organic pollutants (POPs). Monitoring of the compounds on the banned list is required in a variety of environmental matrices. Several pesticides banned by the convention are difficult to analyze by traditional EI+ GC-MS due to significant levels of fragmentation. Therefore, this makes selection of a suitable precursor ion for MS/MS difficult. For multiple reaction monitoring (MRM) analysis, the ability to obtain an intense and specific precursor ion is critical in obtaining low detection limits.
A new ionization technique, Atmospheric Pressure GC (APGC) is presented here as an alternative. Ionization in APGC is analogous to atmospheric pressure chemical ionization (APCI) insomuch as molecular or quasi-molecular ions are produced. APGC is a ‘soft’ ionization technique that results in lower fragmentation. The presence of strong molecular or quasimolecular ions provides ideal conditions for MS/MS analysis.

A Waters Xevo TQ-S coupled to a GC with an APGC Source was operated in MS Scan mode. A suite of pesticides was analyzed with any spectra obtained and compared against the NIST Mass Spectral Library. There are two possible ionization mechanisms, depending on the source conditions: nitrogen charge transfer, producing M+. radical cations: or proton transfer, which produces [M+H]+ ions depending on the source conditions. Dry source conditions will favor nitrogen charge transfer, whereas the presence of H+ ions (e.g. from water or methanol) favor proton transfer.
Organochlorine insecticides, such as endosulfan and cyclodiene-type insecticides, such as aldrin, dieldrin, and endrin, have traditionally proven difficult to analyze in EI+ because of their extensive fragmentation. Due to this fragmentation, the selection of a suitable precursor to product ion transition for MRM analysis is difficult. Figure 2 shows the comparison between the spectrum for endosulfan taken from the NIST 08 Spectral Library and that obtained from APGC. Compared to the EI+ spectrum, the APGC spectrum shows a significant reduction in the fragmentation.
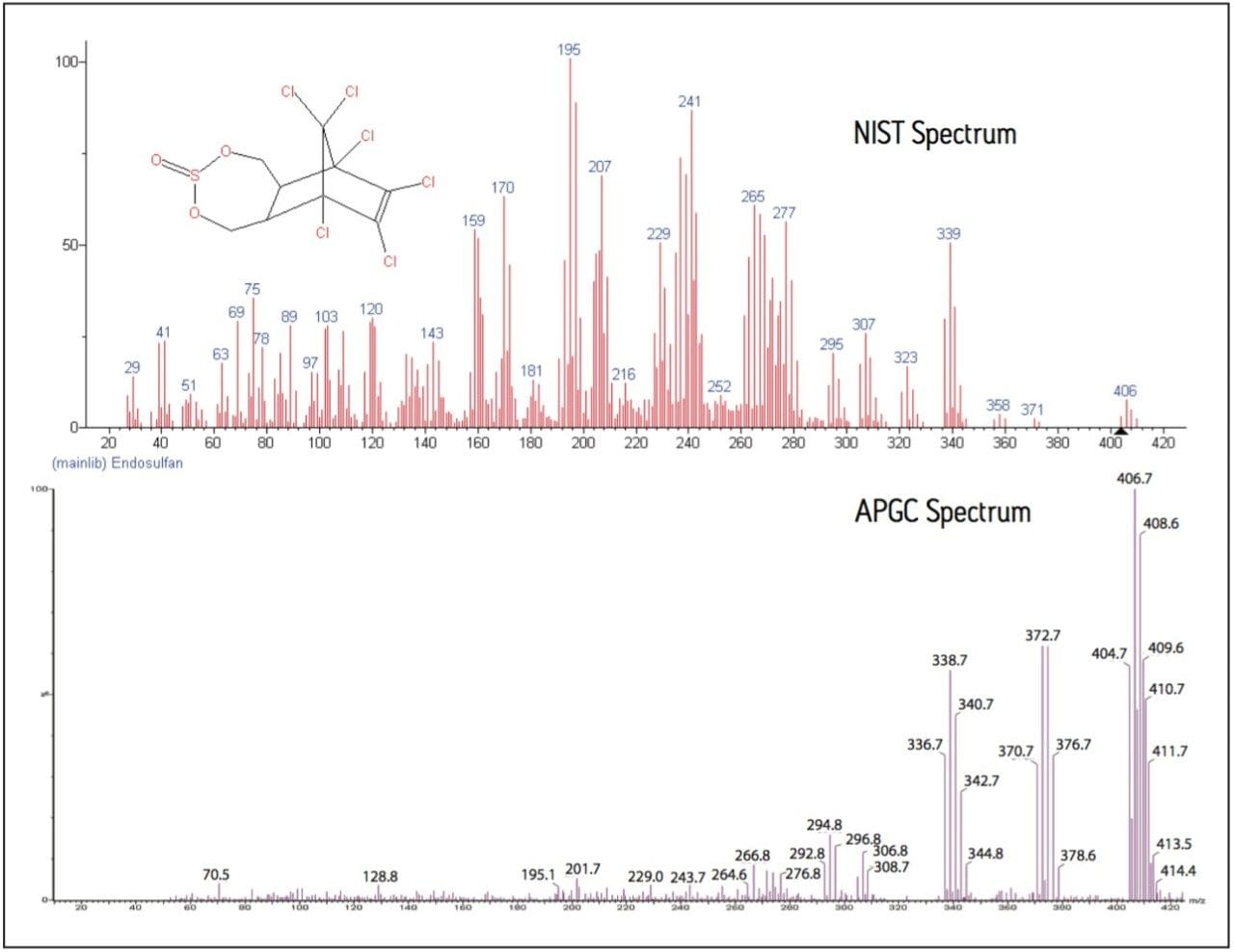
Using APGC, the predominant ion for endosulfan is m/z 407 [M+H]+, rather than the fragment ion m/z 195 in the NIST spectrum. In addition, the ion signal is concentrated into a small number of ions, rather than spread across many fragments. The ions from the APGC spectrum are therefore much more suitable for the precursor ion selection required in MRM analysis. Good selection of the precursor ion is necessary for sensitive and specific MRM traces.
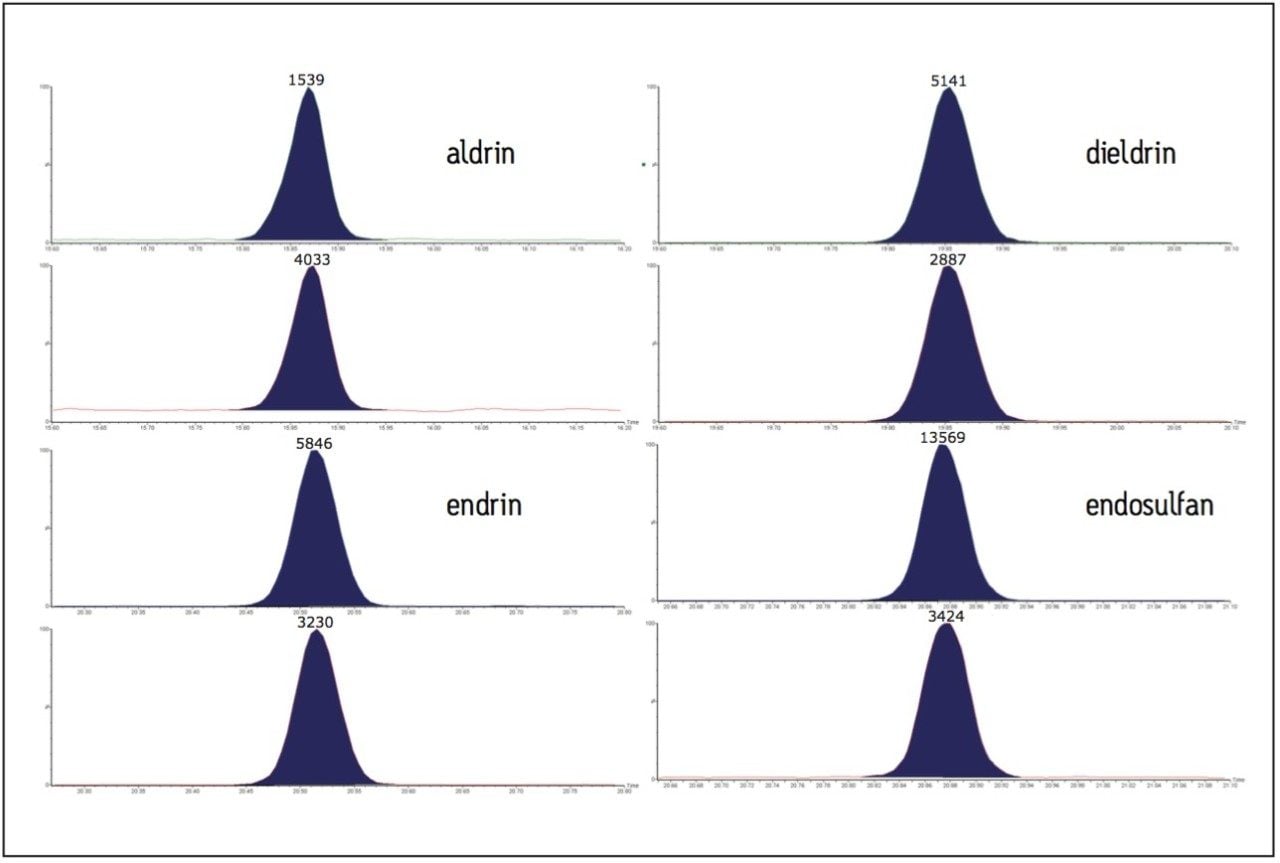
Figure 3 shows two MRM transitions of a low-level standard of endosulfan and also aldrin, dieldrin, and endrin. This demonstrates the application of this technique for the analysis of other traditionally difficult compounds.
720004031, December 2013