Procainamide labeling is a good alternative to traditional 2-AB labeling technique. It follows the same reduction-amination labeling procedure like 2-AB, but it has shown to be a better alternative for fluorescent tagging.
Parallel comparison of two derivatization techniques demonstrated that procainamide labeling of glycan mixture is suitable for UPLC-FLR/MS/MS analysis and it shows excellent chromatographic peak resolution and MS sensitivity.
Characterization of the protein glycosylation profile is of the great importance and is required for various regulatory purposes and production of biopharmaceutical drugs. The released glycan pool is of great complexity and structural heterogeneity, which requires an efficient method of separation and a highly sensitive detection method.
UltraPerformance LC (UPLC) in hydrophilic interaction chromatography (HILIC) separation mode is becoming a routine and widely recognized technique for rapid, efficient, sensitive, and reproducible analysis of 2-aminobenzamide (2-AB) labeled glycans. Even though 2-AB is now the most common glycan labeling reagent, its use is limited by low MS sensitivity. Recent reports on LC-FLR/MS of glycans tagged with procaimanide1 demonstrate this alternative reagent would be advantageous for improving MS ionization efficiency without compromising LC separation.
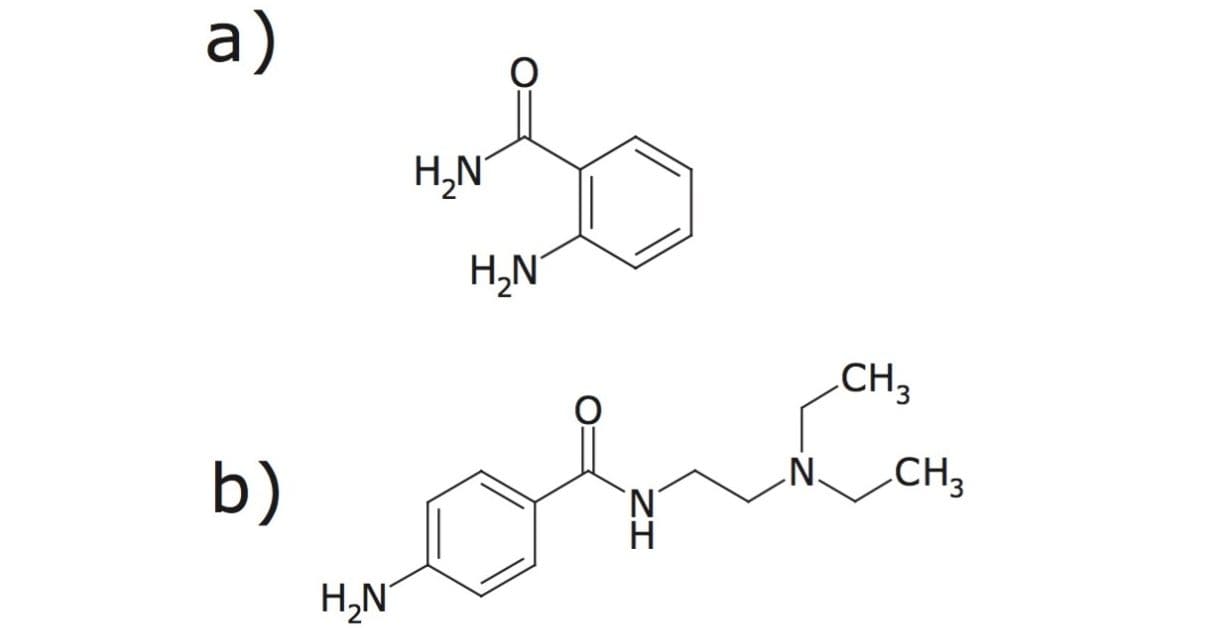
Human IgG N-linked glycan standard library (ProZyme, PN GKLB-005) was fluorescently labeled with either 2-aminobenzamide (2-AB) or procainamide (4-amino-N-(2-diethylaminoethyl) benzamide) reagents (Fig. 1) without prior purification. Fluorescent labeling was performed in solution of 100 μL of glacial acetic acid: DMSO (3:7, v/v) mixture with 11 mg procainamide or 5 mg 2-AB, following by addition of 6 mg of sodium cyanoborohydrate. This labeling reagent was added to 2 μg of human IgG library standards and heated for 4 hours at 65 °C. All samples were reconstituted in acetonitrile/water (1:1) before the injection.
|
LC system: |
Waters ACQUITY UPLC |
|
Detection: |
ACQUITY UPLC Fluorescent Detector, 1 points/s sampling rate. λex 330 nm, λem 420 nm for 2-AB-labeled glycans; λex 308 nm, λem 359 nm for procainamide-labeled glycans |
|
Column: |
Waters Glycan Separation Technology ACQUITY UPLC BEH 1.7-μm, 2.1 x 150 mm (PN 186004742) |
|
Column temp.: |
60 °C |
|
Sample temp.: |
8 °C |
|
Wash: |
Weak wash 75% acetonitrile; strong wash 20% acetonitrile |
|
Mobile phase A: |
50 mM ammonium formate, prepared by titrating ammonium formate solution with formic acid to pH 4.5 |
|
Mobile phase B: |
100% acetonitrile |
|
Gradient: |
72% to 55% B in 45 min, 0.4 mL/min flow rate |
|
MS system: |
Waters Xevo QTof, positive ion mode |
|
Voltages: |
Capillary 3.2 kV, sampling cone 30 V, extraction cone 4 V |
|
Temp.: |
Source temp. 100 °C, desolvation temp. 350 °C |
|
Desolvation gas flow: |
800 L/hr |
|
LockMass calibration: |
CsI 1 mg/mL (water/acetonitrile, 1:1), 5 μL/min flow rate |
|
MS survey data: |
Ecol ≈20 V to 55 V ramp |
MassLynx v. 4.1 Software for control and data acquisition
Derivatization of glycans with 2-AB fluorescent reagent through reductive amination is a routine procedure for the majority of LC-FLR/MS analyses due to the high stability of labeled glycans and their compatibility with MS methods. The sensitivity of fluorescent detection is also suitable for relative quantitation. The sample preparation conditions are well established for efficient labeling, including release of oligosaccharrides and tagging. Commercial labeling kits are also available.
Besides 2-AB, other reagents can potentially be used as fluorescent tags. Labeling with an alternative reagent may improve LC separation and FLR-MS detection. An example of such aromatic amine compound is procainamide, which was reported to have both such advantages and can potentially be used as preferred labeling reagent over 2-AB due to increased MS response.1 Procainamide and 2-AB labeling of IgG glycan standards library was performed in parallel for the same amount of standards mixture.
Qualitatively, FLR response of procainamide-labeled glycans is comparable to that of 2-AB-labeled glycans. The sensitivity of FLR was optimized by tuning λex= 308 nm, λem= 359 nm. Procainamide chromatograms show similar extent of peak separation within the same gradient. The most noticeable difference is that procainamide labeled IgG glycans are more retained under the same chromatographic conditions (Figure 2). Overall chromatographic selectivity is similar between the two labeling reagents.

As described in previous study,1 the advantage of using procainamide over 2-AB is the increased MS ionization efficiency. MS response of 2-AB-labeled G0F, G2F, and G1F is ~15 times lower than that of procainamide-labeled glycan standards (Figure 2 (c, d)). Be aware that MS signal becomes strongly non-linear at high sample concentrations, adversely affecting the shape of MS chromatographic peaks. This should be taken in consideration while doing method transfer from 2-AB labeling to procainamide: the injected sample amount of the latter should not exceed 40 ng of released IgG glycans. The relative ratio of the major MS peaks is similar between procainamide and 2-AB except for intensity spikes of G1F and G1FB.
Procainamide derivatives reveal more minor peaks compare to 2-AB due to its higher MS-ionization efficiencies. Multiple sialylated glycans were detected, they were not observed in the 2-AB glycan MS chromatogram, namely G2S2, G2FS2, and G2FBS2. Also, G0F-N, Man5, G1, and G2B neutral glycans were observed in a procainamide-derivative form but not as 2-AB-derivative (Figure 2(d)). The enhanced MS sensitivity due to procainamide derivatization also improves MS/MS spectra quality. The chromatographic resolution was adequate to analyze each individual peak in the glycan mixture with varying degree of galactosylation and fucosylation in a 45 min gradient.
MS/MS was performed on doubly protonated precursor ions of the five most intense chromatographic peaks (G0F to G2FS1) in order to optimize collision energy (Ecol). For the same species, procainamide-labeled IgG glycans need about 10 to 20% higher Ecol (~ 25-40 V depend on the size of the glycan) compared to Ecol required for fragmenting 2-AB-labeled glycans. No qualitative difference in MS/MS pattern was observed for these two labeling reagents. Figure 3 demonstrates survey spectrum of G0FB component. The entire glycan sequence can be deduced from the series of characteristic ion fragments, which are produced predominantly by glycosidic bond cleavage, so that the method can be potentially applied for the analysis of unknown glycans.

Procainamide labeling proved to be a great alternative to traditional 2-AB labeling technique. It follows the same reduction-amination labeling procedure like 2-AB, but it has shown to be a better alternative for fluorescent tagging.
Parallel comparison of two derivatization techniques demonstrated that procainamide labeling of glycan mixture is suitable for UPLC-FLR/MS/MS analysis and it shows excellent chromatographic peak resolution and MS sensitivity.
The main reason to choose procainamide labeling over traditional 2-AB method is that the former greatly improves the identification of very minor glycans, enhances MS ionization efficiency, allows MS/MS fragmentation for low level species, and therefore, is suitable for variety of de-novo study. Minimal method transfer is required except for sample dilution and slight gradient adjustment if it is needed. It is suitable for both neutral and acidic glycans, since there are no losses of sialylated species upon ESI ionization.
The procainamide derivatization method will be well received by FDA as a part of complete glycoprotein characterization.
720004212, February 2012