
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the ability of high-resolution chromatography to successfully separate an analyte from a complex matrix for bioanalysis, using the ACQUITY UPLC I-Class System with a tandem quadrupole mass spectrometer.
With high-resolution chromatography, fluticasone propionate and salmeterol xinafoate are successfully resolved from chemical interferences in a dried blood spot card, and from endogenous material in precipitated rat plasma samples.
The accurate measurement of bioanalytical samples requires a highly specific and sensitive methodology. LC-MS/MS has become methodology of choice; here the specificity of the methodology relies upon the chromatographic separation of the analyte from the endogenous matrix components and Multiple Reaction Monitoring (MRM) detection in the mass spectrometer.
To achieve a robust assay, it is critical that the analyte being measured is resolved from endogenous matrix peaks, such as phospholipids, that can result in ion suppression and hence irreproducible results.
Achieving optimal chromatographic resolution has always been balanced between speed and resolution for the bioanalyst. Longer separation times deliver superior resolution but reduce throughput. The advent of sub-2-μm particle UPLC Technology allowed
the bioanalyst to improve throughput. However as regulatory guidelines and assay sensitivity requirements have increased, the need for greater chromatographic performance has also increased. Sampling technologies such as dried blood spot and micro-sampling have challenged the detection limits of current methodologies.
The ACQUITY UPLC I-Class System has been specifically designed to deliver extremely high-resolution chromatographic separations. This is achieved by technological advances that allow the system to control dispersion and band spreading while operating at higher system back pressures. These factors combine to allow the bioanalyst to employ longer analytical columns, without compromising throughput, and deliver extremely sharp analytical peaks.
An example of this high-resolution UPLC-MS/MS performance is shown in Figure 1. Here we can see the separation of fluticasone propionate and salmeterol xinafoate from a dried blood spot. The analysis was performed on a 2.1 x 150 mm ACQUITY UPLC C18 1.7-μm Column eluted with 50:85 methanol/aqueous ammonium hydroxide gradient over 10 minutes. The orange trace represents the full-scan positive ion MS data; the MRM trace for salmeterol and fluticasone propionate are represented by the blue trace.
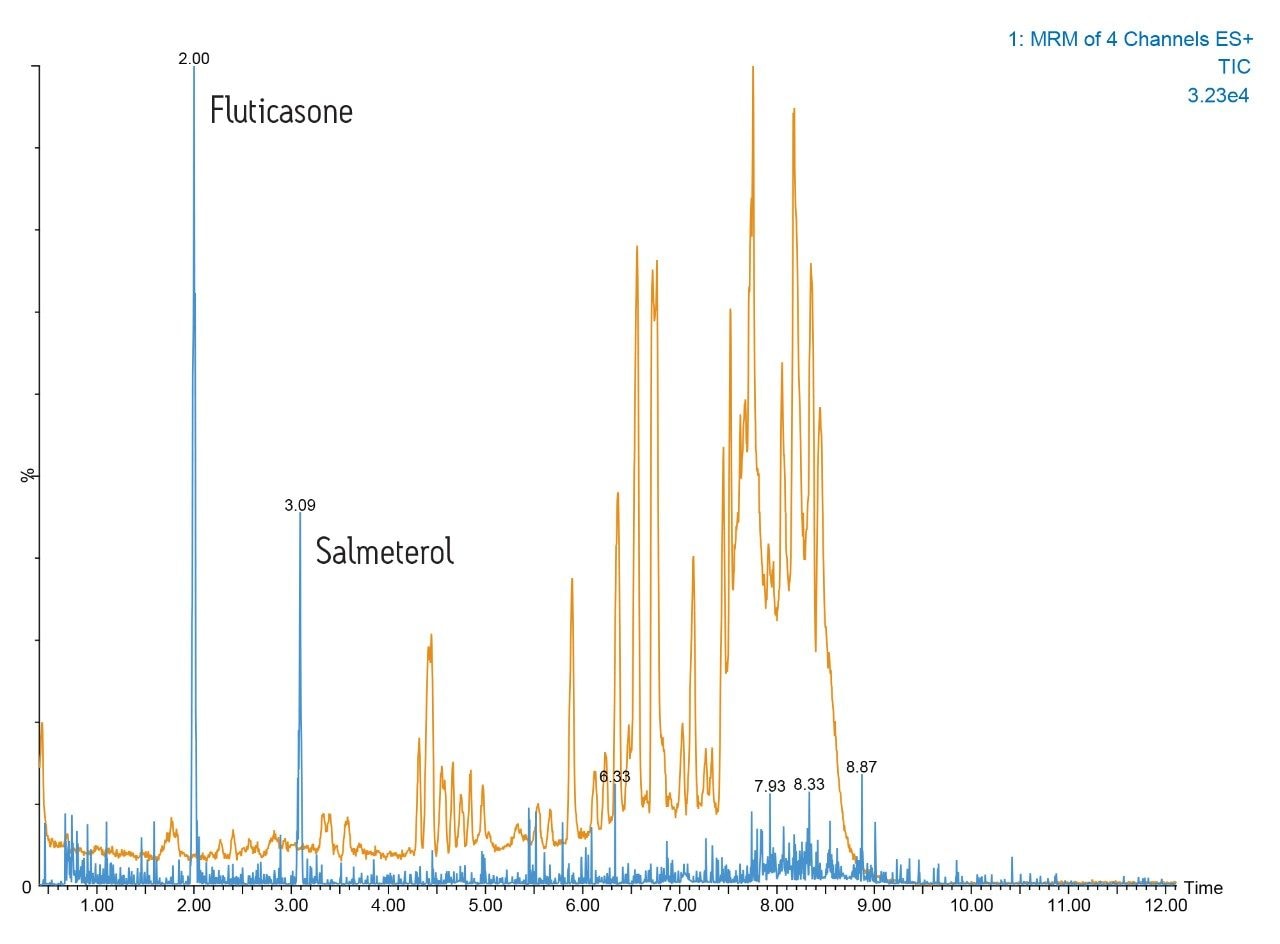
Here we can see that the dried blood spot card contains a large amount of interferences derived from the chemicals added to the card. The highresolution ACQUITY UPLC I-Class System provides very sharp peaks allowing the two pharmaceutical molecules to be quantified without matrix interference.
To further illustrate the high-resolution capability of the system, fluticasone propionate and salmeterol xinafoate were spiked into rat plasma and precipitated with acetonitrile (ratio 2:1). In this example, the analytes were eluted from the column using a 5:95 methanol/aqueous formic acid gradient over 5 minutes, Figure 2.
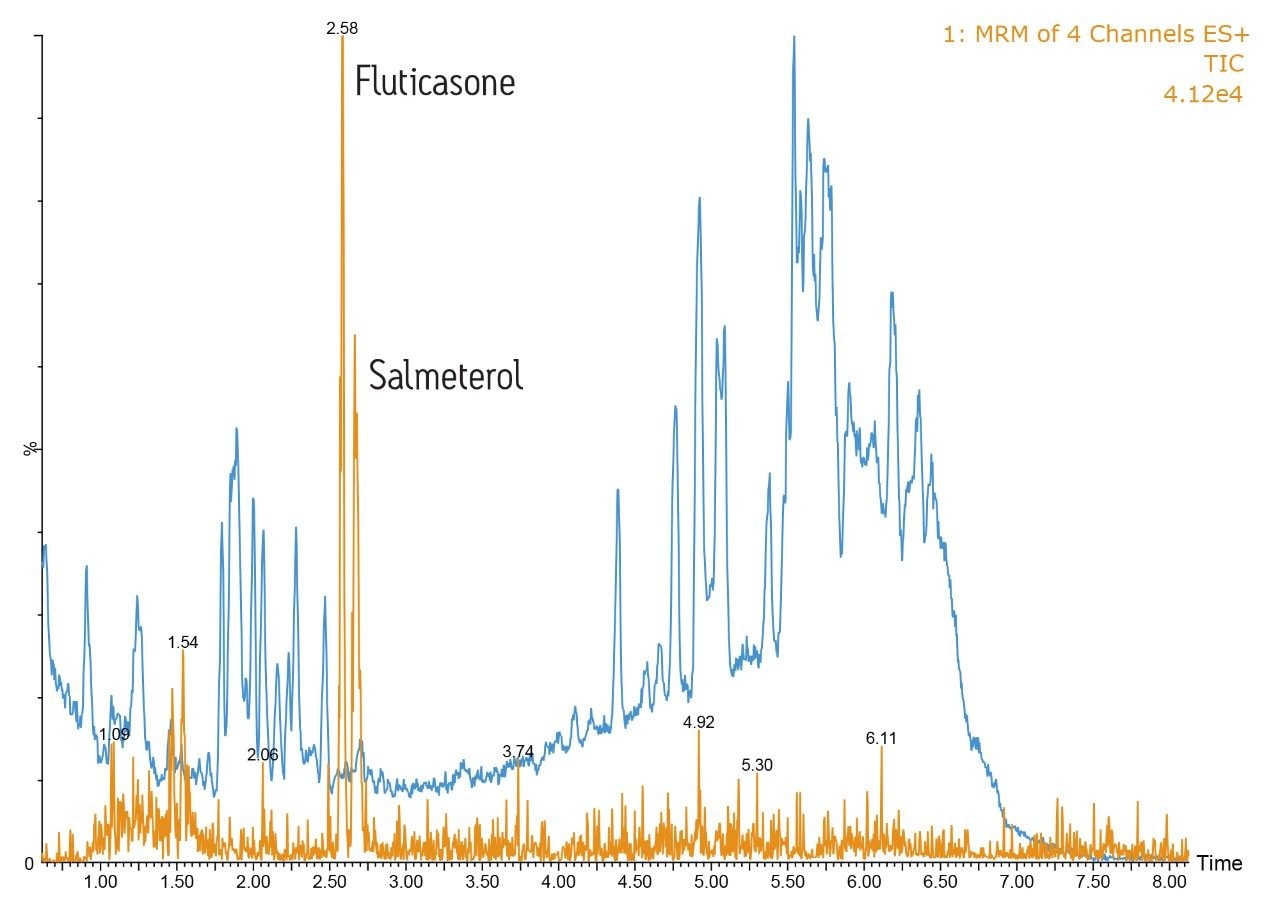
This data illustrates that the background full-scan MS chromatogram, shown in blue, is much more complex than the dried blood spot example. The orange trace represents the MRM signal from the two pharmaceutical compounds, with salmeterol eluting at 2.58 minutes and fluticasone propionate eluting at 2.65 minutes. From the UPLC-MS/MS data it is clear that the ACQUITY UPLC I-Class System provided excellent resolution from the endogenous material in precipitated plasma samples, allowing reliable and accurate quantification of the analytes.
The ACQUITY UPLC I-Class System provides the highest levels of chromatographic performance, providing excellent dispersion characteristics and the ability to use longer columns due to the increased back pressure capability. The use of high-resolution UPLC for bioanalysis delivers sharp analyte peaks for maximum sensitivity and highest resolution that minimizes coelution with endogenous material in the sample. The system’s high back pressure facilitates the use of long, high-resolution columns with run times of just 5 to 10 minutes.
720003936, May 2011