
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrate that fast bioanalytical assays of 45 to 60 seconds that retain high chromatographic fidelity are now possible with the ACQUITY UPLC I-Class System.
In bioanalysis, where the balance between data quality, sensitivity, and productivity must be carefully weighed, the ACQUITY UPLC I-Class System provides unprecedented levels of performance and productivity.
Successful quantitative bioanalysis requires a high-sensitivity analytical methodology. The sensitivity and selectivity of modern LC technologies such as sub-2-μm particle UPLC has resulted in analysis times in the 1.5 to 2 minute range. These high-throughput assays allow several batches to be analysed per day, maximizing instrument utilization and thus their profitability. Even faster analysis times have been possible – but at the expense of chromatographic resolution and assay performance. As assay sensitivity requirements continue to become more challenging – singlefigure pg/mL – the bioanalytical scientist must develop assays that are free from matrix interference and metabolite coelution, while still maintaining the acceptable throughput.
In the last five years, regulatory requirements oon the quality of bioanalytical assays have become more rigorous. Components such as incurred Sample Reanalysis (ISR), metabolites In Safety Testing (MIST), and matrix factor are all becoming more prominent considerations for the bioanalyst.
To meet these requirements, it has been necessary to improve the sample preparation process and to develop more selective and specific separations methods. This results in increased analysis times. Analytical systems are now being challenged to execute high-throughput methods that require low-volume chromatographic instrumentation, a rapid injection-to-injection autosampler, and fast detection capabilities as well as the ability to address the backpressure developed by modern sub-2-μm chromatography columns.
The ACQUITY UPLC I-Class System has been specifically designed to deliver rapid gradients with low system delay volume and a fast, flow-throughneedle autosampler. This, combined with its binary solvent manager’s ability to deliver high-fidelity gradients extremely rapidly, makes the ACQUITY UPLC I-Class the ideal chromatographic solution for bioanalysis.
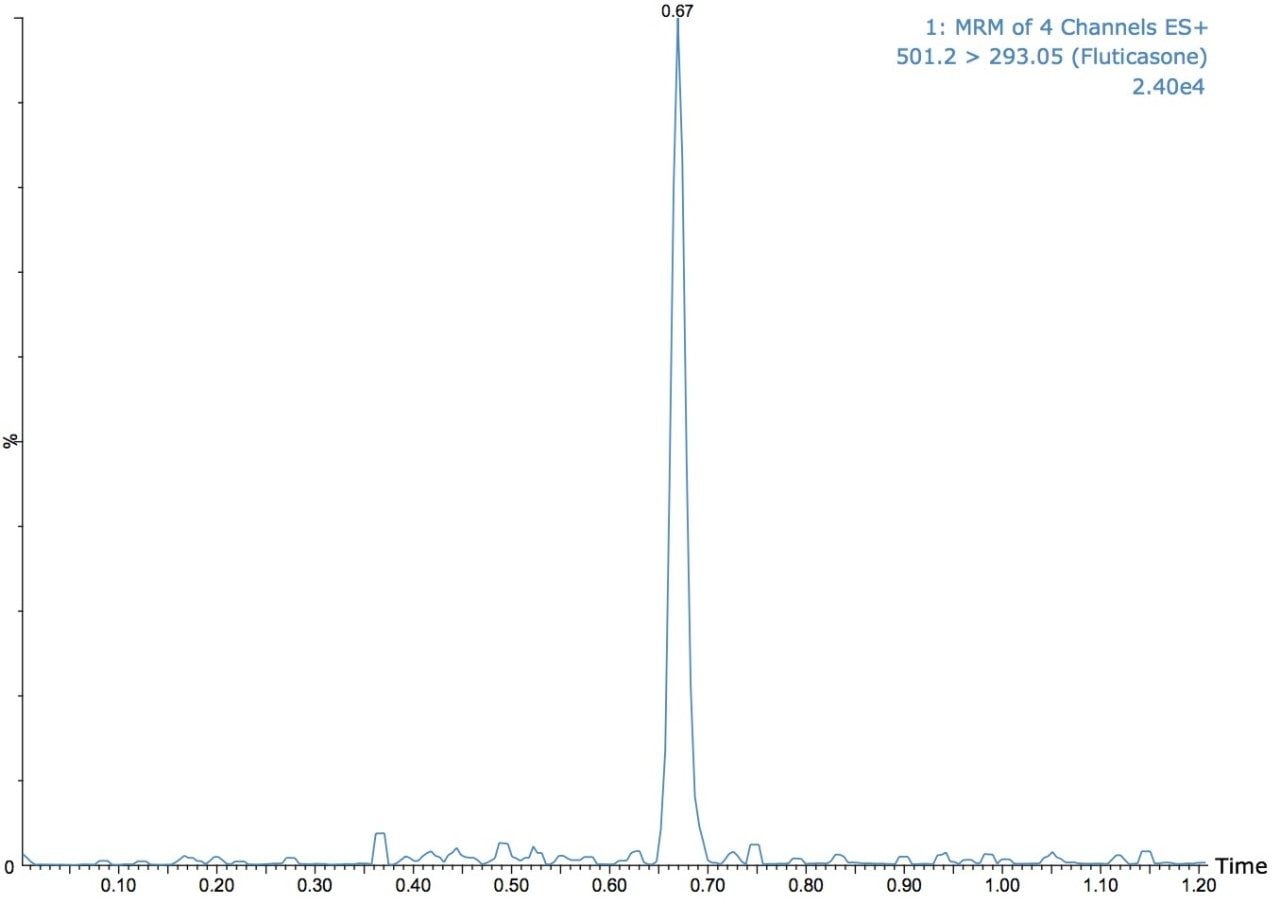
The UPLC-MS/MS analysis shown in Figure 1 illustrates the quantification of fluticasone propionate. The separation in Figure 1 shows the use of a 60-second gradient. The fluticasone propionate peak elutes at a time of 0.67 min. The effect of reducing the gradient time from 90 seconds to 45 seconds, in these examples the same chromatography column is employed a 2.1 x 50 mm ACQUITY UPLC C18, 1.7-μm column with a 50:85 methanol/aqueous ammonium hydroxide gradient at 500 μL/min.
The ACQUITY UPLC I-Class System provides the highest levels of chromatographic performance, providing excellent dispersion characteristics and the ability to use longer columns due to the increased back pressure capability. The use of high-resolution UPLC for bioanalysis delivers sharp analyte peaks for maximum sensitivity and highest resolution that minimizes coelution with endogenous material in the sample. The system’s high back pressure facilitates the use of long, high resolution, columns with run times of just 5 to 10 minutes.
720003937, May 2011