In this application note crude synthetic peptide samples were separated on preparative Atlantis dC18 columns. Fraction collection was triggered by the mass-to-charge (m/z) ratio of the peptides, which specified the target peptide in the collected fraction to increase the fraction purities. Mass-directed peptide purification was more robust than UV-based collection under overloading conditions, and increased the fraction purities. LC–MS/MS analysis was further employed to identify the impurities in the crude synthetic peptide sample. This study demonstrates high mass loading, selective purification, high recoveries and purity, and the capability of utilizing mass-directed fractionation for purifying peptides on preparative RPLC columns.
Excellent efficiency, high mass loading, and ease of scale-up
Purifying crude samples of synthetic peptides is of great interest to chromatographers. The Atlantis dC18 material was designed with the best combination of pore size, ligand density, and ligand type for polar and non-polar compounds. We first developed sep-arations of a crude synthetic peptide sample on an analytical dimension Atlantis dC18 column. We then scaled-up the sep-aration to a semi-preparative dimension to demonstrate the utility of these columns for synthetic peptide purification. Finally, LC–MS-MS analysis was applied to determine the peptide sequence of each impurity in the chromatograms.
Analytical separations were performed on an Atlantis dC18 4.6 x 100 mm, 5 µm column. Preparative separations were performed on an Atlantis dC18 10 x 100 mm, 5-µm column. The peptide sample was synthesized by Cell Essentials, Inc. (Boston, Massachusetts). The crude synthetic peptide sample was dissolved in 0.2% formic acid. The mobile phases consisted of A: water with 0.2% formic acid, and B: acetonitrile with 0.2% formic acid. Gradient methodology is listed in the figure caption. Flow rates were 1.0 mL/min for the 4.6-mm i.d. column and 4.72 mL/min for the 10-mm i.d. column. All experiments were run on the Waters AutoPurification System, which consists of a Waters 2525 Binary Gradient Module, a Waters 2767 Sample Manager, a Waters 2996 Photodiode Array Detector, and a Waters ZQ Mass Spectrometer. The LC–MS/MS experiment was run on the Waters Q-TOF Micro Mass Spectrometer. All experiments were conducted at ambient temperatures. MS analysis was carried out in positive ion mode. The capillary and cone voltages were set at 3.5 kV and 25 V, respectively. The source and desolvation temperatures were set at 100 °C and 250 °C, respectively. Nitrogen was used as the cone and desolvation gases with flow rates of 50 L/h and 250 L/h, respectively.
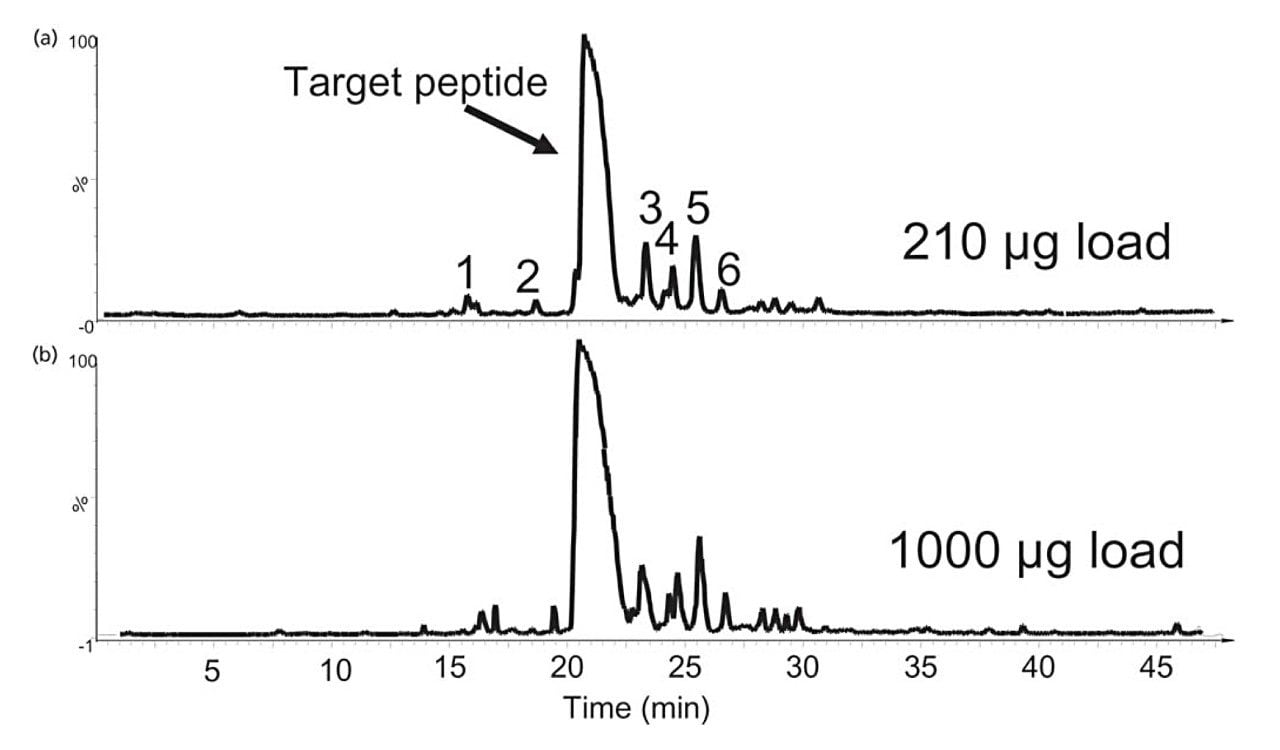
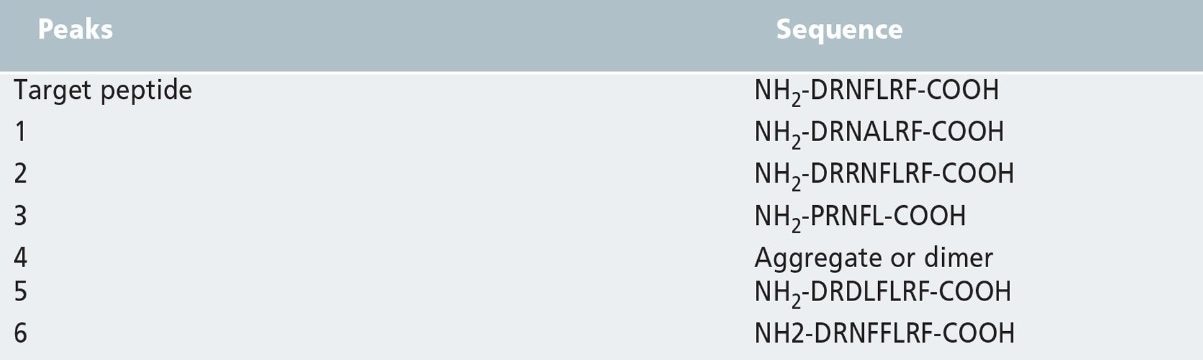
The separation of the crude synthetic peptide sample on the analytical column is shown in Figure 1a. The total load is 210 µg. The main peak is the target peptide. This peptide has the desired sequence of NH2-DRNFLRF-COOH with a molecular weight of 967. The mass load was proportionally scaled-up and run on the semi-preparative column (shown in Figure 1b). Note the direct scale up, excellent peak shapes and total mass load of 1000 µg. LC–MS/MS analysis was run to identify the sequence information of each impurity in the chromatograms. The sequences are listed in Table I.
Atlantis dC18 RPLC columns are useful tools for retaining and separating synthetic peptides under highly aqueous mobile phase conditions. Since Atlantis dC18 columns are fully LC–MS compatible, they can be used in a mass-directed chromatographic purification system. Atlantis dC18 preparative columns provide excellent efficiency, high mass loading, and ease of scale-up.
WA31810, February 2004