High Throughput Solid Phase Extraction and LC-MS/MS Determination of 27 Multiclass Synthetic and Natural Steroidal Hormones and Sterols in Untreated Wastewater Samples Using APCI
Chaitanya Devireddy1, Bhaskar Karubothula1, Rizwan Shukoor1, Raghu Tadala1, Dnyaneshwar Shinde1, Dr Parth Gupta1, Dr Wael Elamin1, Dr Grzegorz Brudecki1, Samara Bin Salem2, P.M.N. Rajesh3, Jesus Gomez-Mares3, Claudia Rathmann3
1 RASID ADQCC- operated by M42, Abu Dhabi, UAE
2 Abu Dhabi Quality and Conformity Council, Abu Dhabi, UAE
3 Waters Corporation, United States
Published on May 23, 2025
Abstract
A robust high throughput single analytical method is needed for detection, quantification, and identification of multiclass synthetic and natural steroidal hormones present in untreated wastewater samples with lower sample processing volumes. This application note introduces a streamlined analysis technique without derivatization, utilizing ultra-high-performance liquid chromatography coupled with atmospheric pressure chemical ionization tandem quadrupole mass spectrometry (UPLC™–APCI-MS/MS). Unlike conventional methods, this approach eliminates the need for time-consuming derivatization and labor-intensive liquid-liquid extraction (LLE). In this study, untreated wastewater samples spiked with standard steroidal hormones were subjected to extraction using an Oasis™ HLB Solid-Phase Extraction (SPE) protocol. Subsequently, the samples were analyzed using the ACQUITY™ UPLC I-Class Plus System coupled with the Xevo™ TQ-XS Tandem Quadrupole Mass Spectrometer. Good peak shape and stable retention times were achieved with an ACQUITY Premier BEH™ C18 Column. The performance of the method was evaluated by in-house method validation, spiking screened blank wastewater matrix samples. Results from method validation showed that the trueness of the method was between 74% to 103% at compound specific lowest quality control (LQC) level (n=18). Close agreement was observed with the repeatability, within laboratory reproducibility, all being <13% RSD. The method has demonstrated to be sensitive, specific, accurate, and appropriate for determining multiclass steroidal hormones (which included 27 analytes) in wastewater matrix, collected from 100 locations every day. It can also be used to support AI-based surveillance programs, research studies on the environmental impact, and the estimation of human and aquatic exposure in the Gulf region.
Benefits
- Automated sample preparation using Oasis HLB SPE allowing low sample volume and resulting in shorter time to process the sample and reduced number of steps for manual handling errors to be introduced
- Use of an ACQUITY Premier Column for good resolution and peak shape of 27 steroidal hormones minimizing coextracted interferences, improving ionization efficiency and automatic data processing
- High analytical sensitivity by using the Xevo TQ-XS equipped with APCI ion source permitting linear, accurate, and precise quantitative performance, achieving LLOQs of up to 0.2 ng/mL
Introduction
In recent years, the occurrence of natural and synthetic endocrine-disrupting steroids as pollutants in the environment has caused widespread concern. Both natural and synthetic androgens, estrogens, progestins and sterols are widely consumed as oral contraceptives, for hormone replacement therapy, for menopausal disorder treatments, and as food supplements to reduce low density lipoprotein (LDL) cholesterol levels. Thus, they arrive in wastewater, and from there into environmental water bodies such as rivers, lakes, and the sea. The continuous release of these compounds combined with the fact that they are designed to have a biological effect at low concentrations, means there is a need to develop sensitive and robust methodologies for their determination.1,2,3
The composition of the wastewater matrix is complex and varies greatly based on location, time of day, and seasonal changes. It contains a wide range of chemical substances originating from various sources such as households, industries, and hospitals. Wastewater is not just a mixture of compounds but also contains a complex matrix of dissolved and suspended solids, salts, proteins, lipids, and other organic and inorganic substances in the form of salts and ions. Analyte signals may be suppressed or enhanced as a result of these matrix components interfering with sample extraction, chromatographic separation, and mass spectrometry ionization.
Existing wastewater determinations are restricted to a narrow section of steroidal hormones analysis with higher wastewater sample processing volumes ranging from 250 mL to 1000 mL. Those methods lack specificity and tend to be highly variable at lower steroid concentrations. The high processing volumes of these conventional monitoring approaches do not effectively track the daily excretion of the target analytes in wastewater. Alternatively, methods such as EPA 1698 GC-HRMS and EPA1694 LC-MS/MS require more laborious extraction and purification steps, as well as derivatization for analysis. These protocols are impractical for high throughput analysis which is necessary for continuous wastewater surveillance across multiple sites daily.4,5
Compared to common pharmaceutical residues, the analysis of steroidal hormones provides unique challenges due to various factors such as a large range of logP values (ranging from 2.50 to 9.40), poor ionization characteristics, and low sensitivity requirements. In our study the intricate composition of untreated wastewater matrices, the requested high daily sample number and the goal to include five different classes of synthetic and natural steroidal hormones (in total 27), namely progestogens, estrogens, androgens, sterols, and phytosterols, adds to the complexity of the analysis. To overcome these challenges a high throughput SPE method in conjunction with UPLC and APCI-MS/MS was developed to quantify at lowest limit of quantification (LLOQ) levels using a processing volume of only 5 mL. Even with the low sample volume, the obtained LLOQs are still lower than the documented EPA methodologies, which employ processing volumes from 500 mL to 1000 mL.
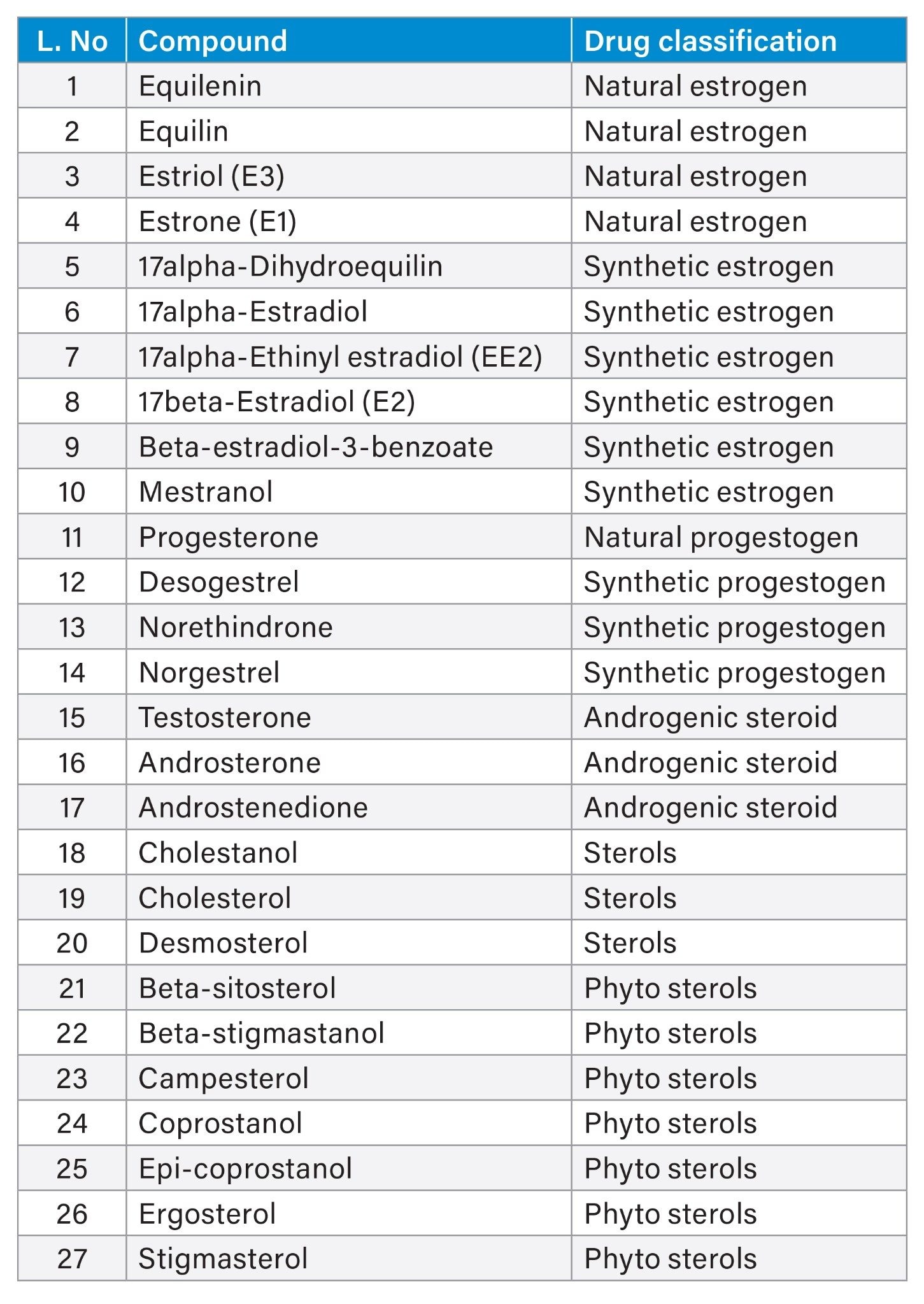
Table 1 details the drug classification of all 27 analytes examined in the study
Experimental
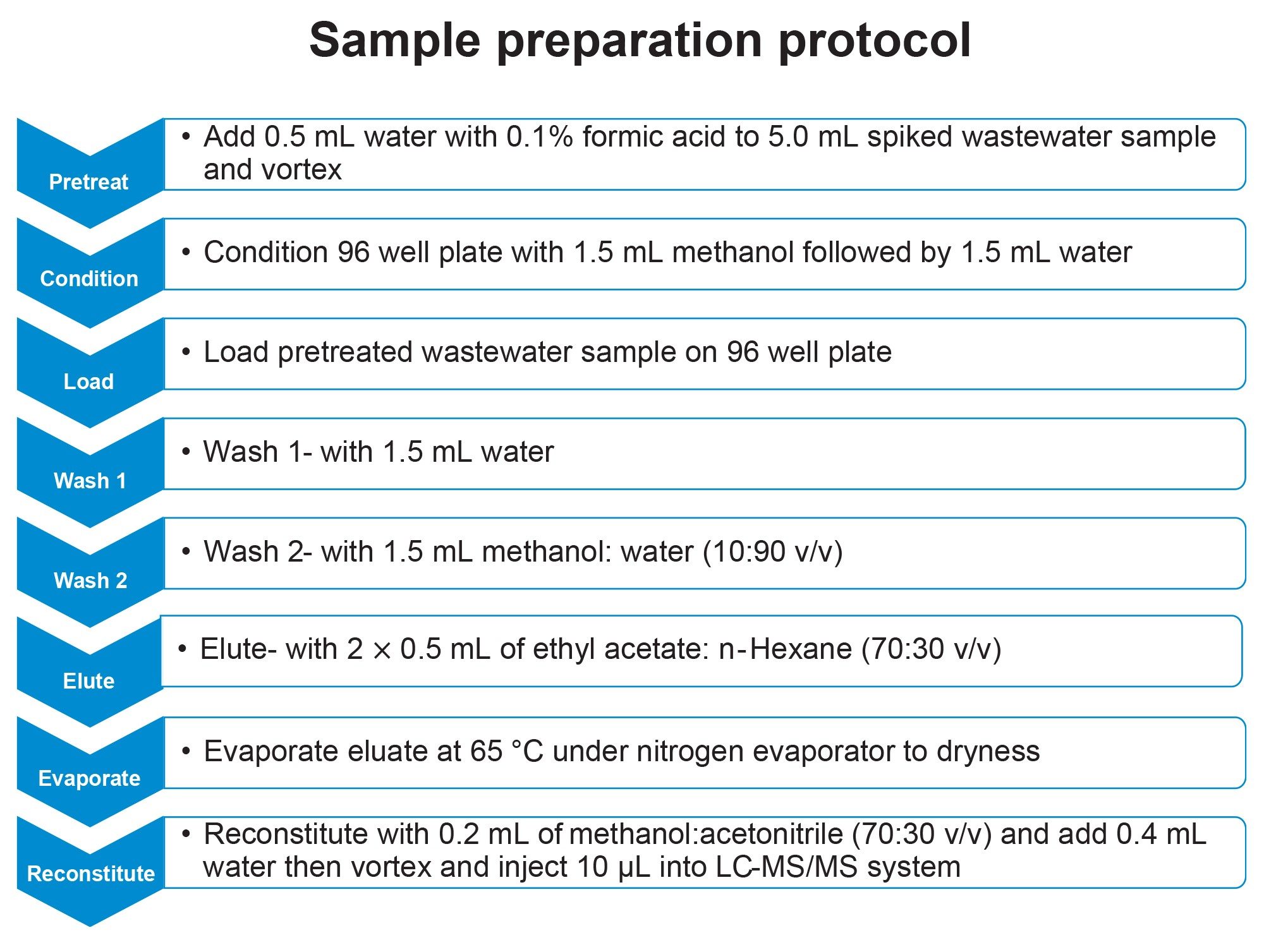
Sample Preparation
Untreated wastewater was screened and used as blank matrix for the study. Calibration standards and quality control (QC) samples were prepared by spiking a mixture of 27 steroidal hormones from an intermediate stock mix into 5 mL of blank matrix at five compound specific concentration levels (refer Table 4). Calibration standards were prepared in duplicate, while six replicates of each QC level, independent of calibration standards, were prepared. The samples were acidified by adding and mixing 0.5 mL LC-MS grade water containing 0.1% formic acid. The acidified spiked wastewater samples were extracted using the Oasis HLB 96-well plate (p/n: 186000679). The detailed extraction protocol is shown in Figure 1. The combination of ethyl acetate and n-hexane as elution solvent increased the elution strength for highly nonpolar analytes like cholesterol.
LC Conditions
|
LC system: |
ACQUITY UPLC I-Class Plus System |
|
Column: |
ACQUITY Premier BEH C18 Column, 130Å, 1.7 µm, 2.1 x 100 mm (p/n: 186009453) |
|
Column temperature: |
65 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
10 µL |
|
Mobile phases: |
A: 0.2 mM ammonium fluoride in water B: Methanol |
|
Purge and wash solvent: |
1:1:1:1 Acetonitrile:Methanol:Isopropanol:Water with 0.1% Formic acid |
|
Seal wash: |
Water:Methanol (95:5 v/v) |
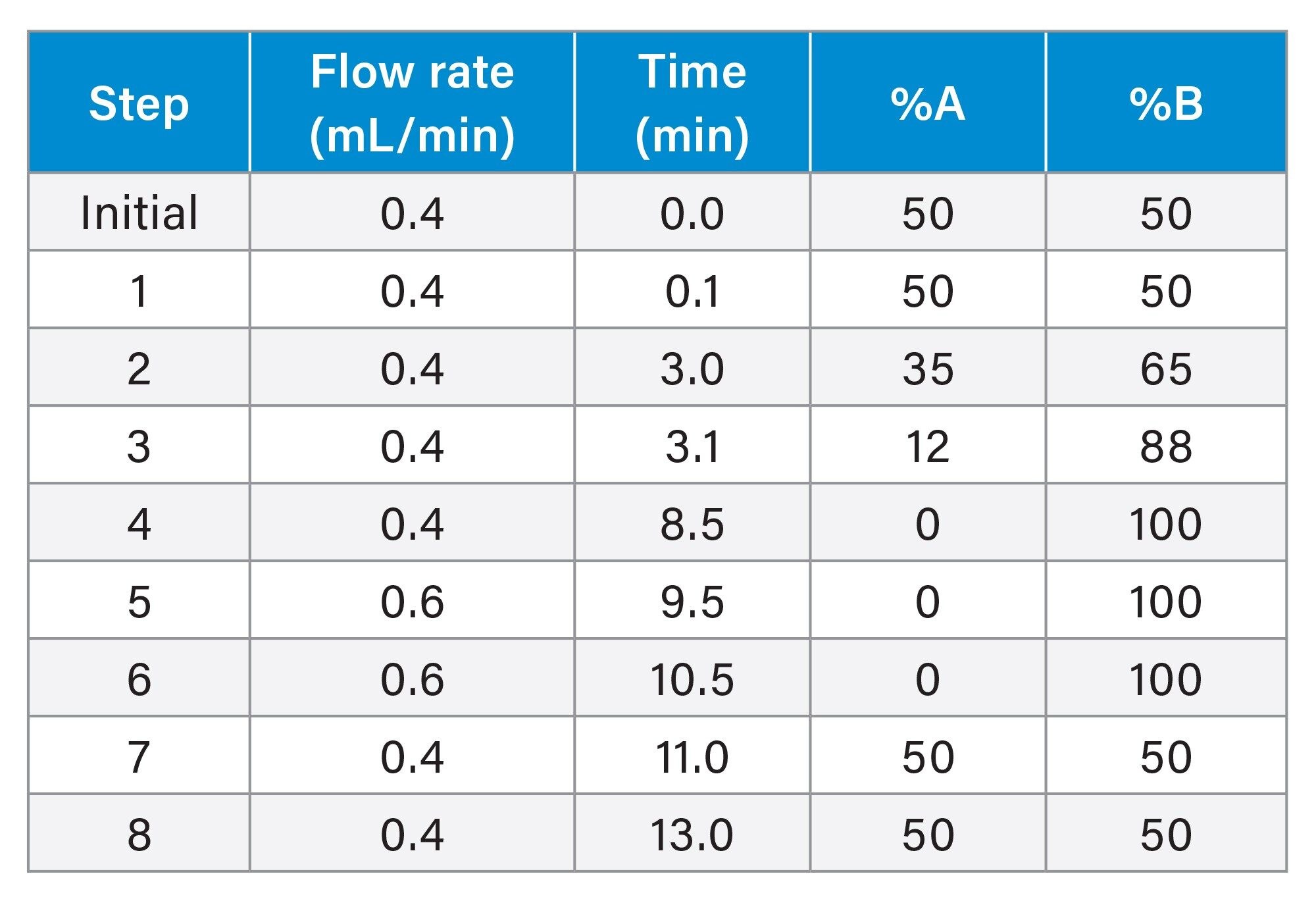
Gradient Table
MS Conditions
|
Mass spectrometer: |
Waters Xevo TQ-XS Tandem Quadrupole |
|
Resolution: |
MS1(0.75 FWHM) |
|
Ionization mode: |
APCI positive/negative polarity switching |
|
Corona voltage for +ve: |
0.8 kV |
|
Corona voltage for -ve: |
1.2 kV |
|
Source temperature: |
150 ºC |
|
Desolvation temperature: |
550 ºC |
|
Cone gas flow: |
150 L/h |
|
Desolvation gas flow: |
1000 L/h |
Data Management
|
MS acquisition software: |
MassLynx™ (v 4.2) |
|
Quantitation software: |
TargetLynx™ |
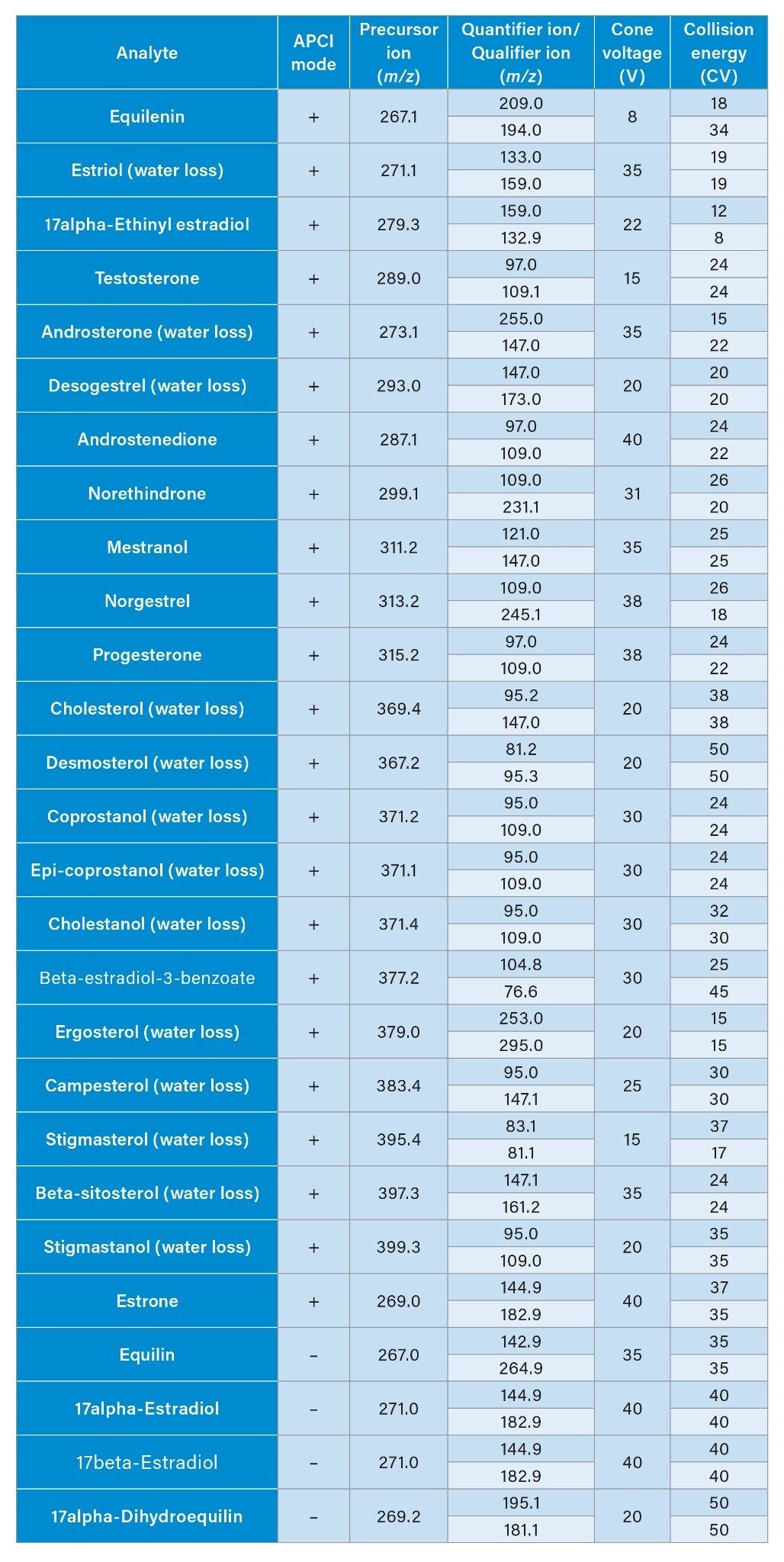
During the optimization of the MRM transitions we observed water loss precursors for some of the steroidal hormones. The use of fragments produced from water loss precursors, in combination with enhanced clean-up by SPE, increased the specificity for all 27 analytes. Table 3 lists the MRM transitions, including the optimized water loss precursors, with MS conditions utilized for quantification.
Results and Discussion
Chromatography
During initial method development, several reversed-phase columns were evaluated for overall chromatographic performance (retention, peak shape and resolution from endogenous interferences, etc.). The best chromatographic performance for all 27 steroidal hormones was achieved using the ACQUITY Premier BEH C18 Column. The optimized LC-method provided sufficient separation of isomers like 17alpha- and 17beta-estradiol but also an acceptable run time for high throughput. The column further demonstrated retention complying with the requirements of the EPA 1694 method, exhibited Gaussian chromatographic peak shapes and good retention time stability throughout the validation. Figures 2 and 3 display overlay chromatograms for 27 analytes and 10 ISTD’s, respectively, spiked and extracted at LLOQ levels in wastewater samples.
A linear flow gradient (Table 2) with an increased flow rate at 100% eluent B improved the rinsing of the column and reduced carryover for late eluting highly nonpolar compounds.
Sensitivity and linearity range
Required LLOQs, Upper Limits of Quantification (ULOQ) and correlating linearity ranges were determined by estimating the concentration ranges of each analyte in routine wastewater samples taken at 100 different locations in the region. Based on the higher and lower detection concentrations found in the wastewater samples of the region, five distinct linearity ranges were defined and LLOQ and ULOQ concentrations established for all 27 analytes. These five linearity ranges were 0.2–3 µg/L, 1–15 µg/L, 10–150 µg/L, 20–300 µg/L, and 40–600 µg/L. Table 4 assigns the respective linearity range for each analyte.
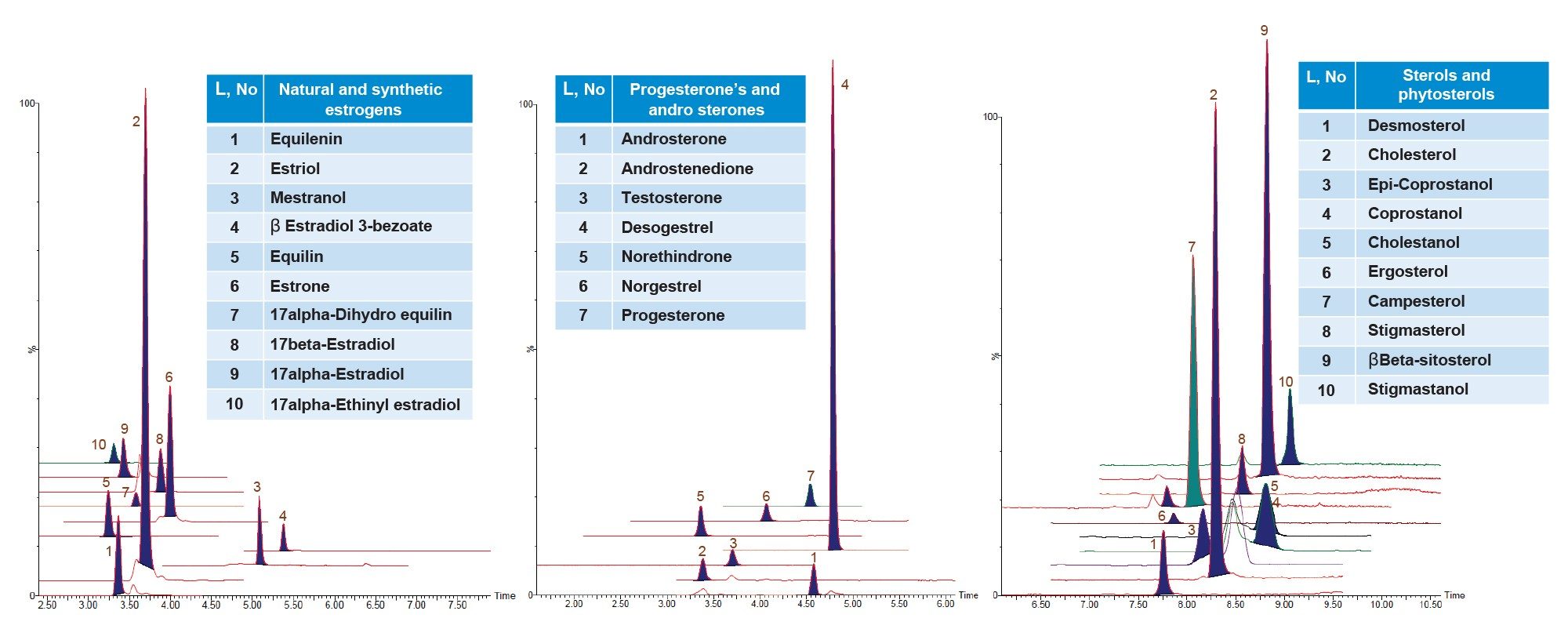
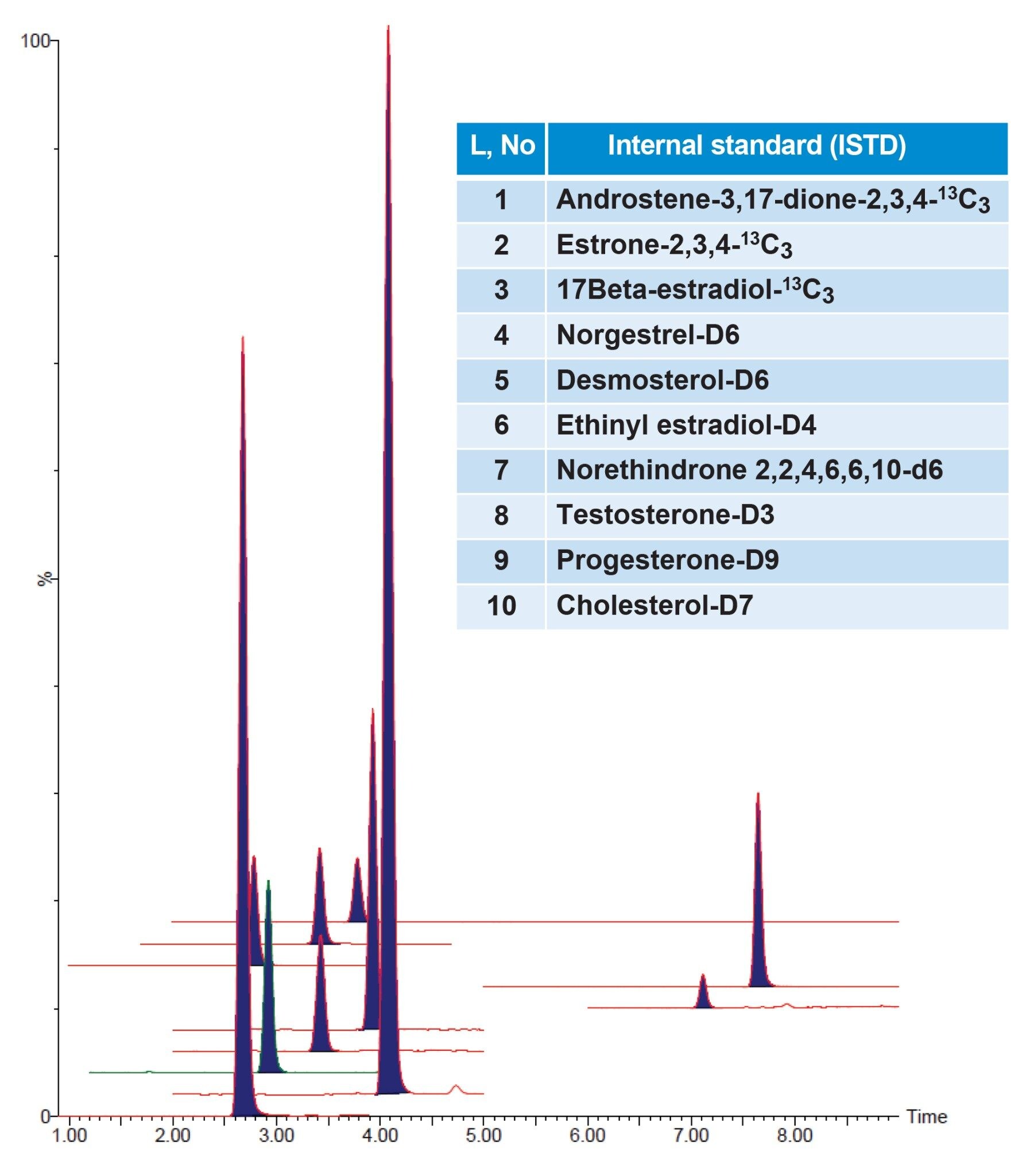
Method Validation
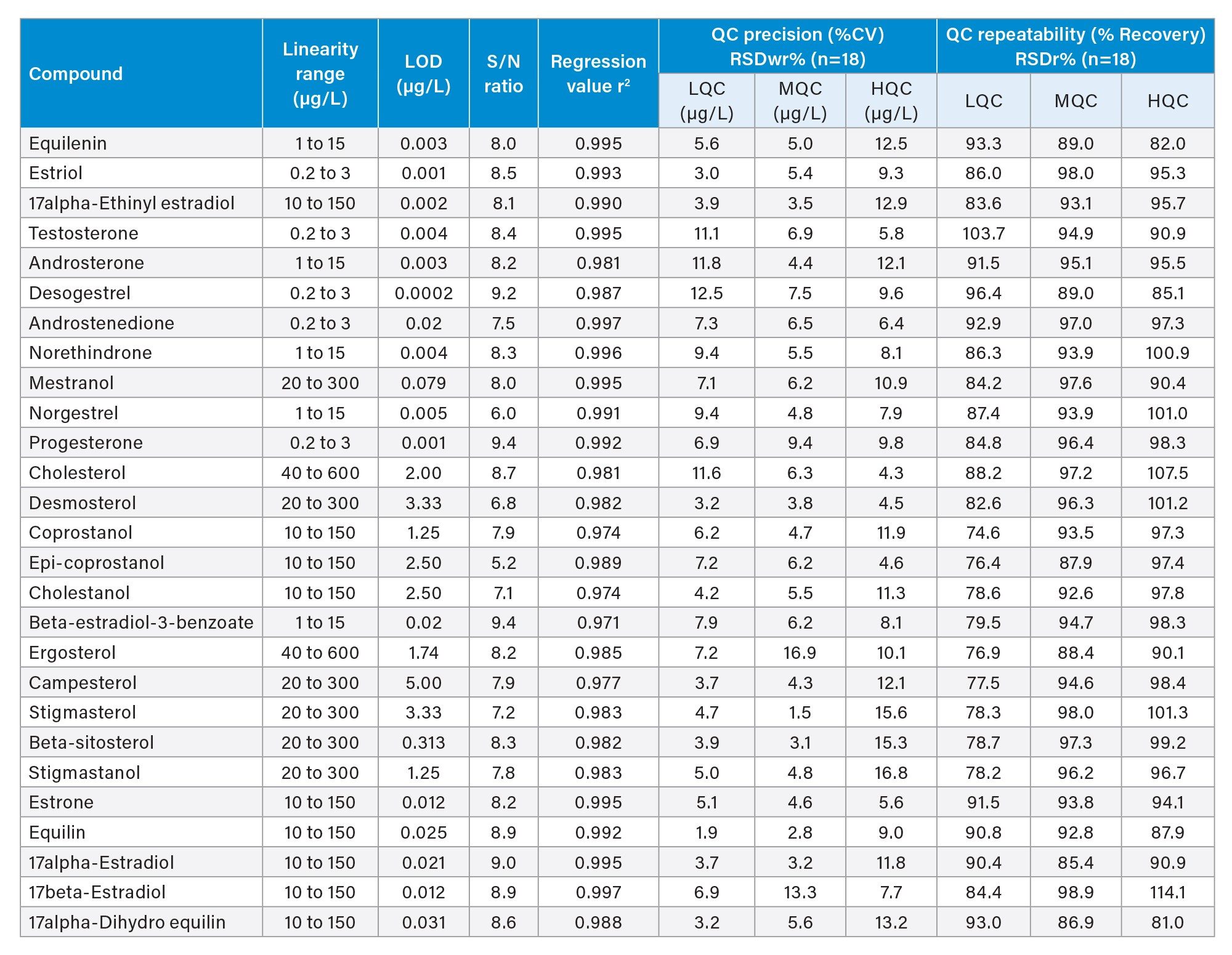
Single laboratory validation was performed by replicate analysis of spiked wastewater samples. The following factors were assessed: selectivity, sensitivity, calibration graph characteristics, trueness, within-laboratory repeatability (pooled RSDr) and within-laboratory precision (RSDwr), also called intermediate precision. For the single laboratory validation, trueness, pooled RSDr and RSDwr were determined from the replicate analysis (n=18) of spikes prepared at three different QC concentrations which are independent from calibration concentrations and named as LQC, MQC & HQC in previously screened untreated wastewater blank matrix. After adjusting for recovery using the appropriate internal standards, all concentrations were calculated using the peak area ratio by pooling the findings from all Day 1, Day 2, and Day 3 results.
With in laboratory validation results
The results demonstrated good accuracy for the quantification of all 27 analytes in untreated wastewater. Table 4 shows a summary of the mean measured recovery values, with associated repeatability and precision.
Conclusion
This application showcases the development of a robust LC-MS/MS method for the accurate quantification of 27 steroidal hormones extracted from untreated wastewater. With the described method, the sample processing volume was reduced from 1000 mL to 5 mL by employing a SPE protocol with Oasis HLB 96-well plates, in combination with the excellent chromatographic separation provided by an ACQUITY UPLC I-Class Plus System and the high sensitivity of the Xevo TQ-XS Mass Spectrometer. Lowering the sample volume by a factor of 200 enabled the method to be used for high throughput analysis. Although only 5 mL of sample processing volume is used, the achieved sensitivity is better than required against the EPA regulations, realizing impressive LLOQ values ranging from 0.2 µg/L to 40.0 µg/L across five distinct linearity ranges that were empirically determined depending on the sampling site.
Moreover, this developed method has undergone successful validation in accordance with EPA guidelines. Its efficiency is further demonstrated by its deployment in high throughput routine sample analysis, enabling the processing of up to 100 samples per day. This advancement represents a significant step forward in real time high throughput daily wastewater surveillance programs, offering a comprehensive and efficient solution for the quantification of steroidal hormones without compromising sensitivity, accuracy, or precision in accordance with international regulatory guidelines requirements
References
- González, Alba, and Vı́ctor Cerdà. “Development of an Automatic Sequential Injection Analysis-Lab on Valve System Exploiting Molecularly Imprinted Polymers Coupled with High Performance Liquid Chromatography for the Determination of Estrogens in Wastewater Samples.” Talanta, vol. 209, 1 Mar. 2020, pp. 120564–120564, https://doi.org/10.1016/j.talanta.2019.120564. Accessed 12 Oct. 2023.
- González, Anelisa, et al. “Steroid Hormones and Estrogenic Activity in the Wastewater Outfall and Receiving Waters of the Chascomús Chained Shallow Lakes System (Argentina).” Science of the Total Environment, vol. 743, Nov. 2020, p. 140401, https://doi.org/10.1016/j.scitotenv.2020.140401.
- Merlo, Francesca, et al. “HPLC-MS/MS Multiclass Determination of Steroid Hormones in Environmental Waters after Preconcentration on the Carbonaceous Sorbent HA-C@Silica.” Arabian Journal of Chemistry, vol. 13, no. 3, 1 Mar. 2020, pp.4673–4680, www.sciencedirect.com/science/article/pii/S1878535219301297, https://doi.org/10.1016/j.arabjc.2019.10.009. Accessed 6 Jan. 2023.
- Sapozhnikova, Yelena, et al. “Analysis of Selected Natural and Synthetic Hormones by LC-MS-MS Using the US EPA Method 1694.” Analytical Methods, vol. 3, no. 5, 2011, p. 1079, https://doi.org/10.1039/c0ay00748j. Accessed 1 Sept. 2020.
- Method 1698: Steroids and Hormones in Water, Soil, Sediment, and Biosolids by HRGC/HRMS. 2007.
720008657, March 2025