The advent of more robust chromatographic materials has facilitated the use of a wider range of method conditions. However, depending on the chemical properties of the analyte, sample degradation may occur during the chromatographic separation. This may be caused by various factors including the use of high temperature, stability in certain mobile phases, pH conditions, or even interactions with the stationary phase and columns.
On-column degradation is more commonly reported for biopharmaceuticals rather than for small molecules. These larger biomolecules can undergo conformational changes and on-column degradation with the stationary phase and LC conditions, respectively. While reports of on-column degradation of small molecules may be rarer, significant cases have been observed. Instances of on-column degradation of amino compounds have been encountered, and their degradation has been found to be exacerbated using high pH mobile phases.
This application note demonstrates that small molecule amines can indeed undergo on-column oxidation when separated with conventional stainless-steel columns. However, this degradation can be mitigated with the use of ACQUITY Premier Columns. ACQUITY Premier Columns feature MaxPeak High Performance Surfaces, which provide a barrier against metal-analyte interactions. This barrier, based on hybrid organic-inorganic silica, can also help protect metal LC surfaces from corrosion, which can further exacerbate analyte degradation. These results demonstrate that the MaxPeak Premier Technology can reduce on-column, metal catalyzed reactions like oxidation. ACQUITY Premier Columns should thereby be considered for use in LC work, purity measurements, and impurity profiling or wherever analytical artifacts might undermine the value of an assay.
Interactions between analytes and the chromatographic system can be a problem for several reasons. In one case, sample adsorption to the stationary phase or surfaces of the LC system can result in low recovery and reproducibility. In another case, analytes can undergo reactions within the column. It is known that peptides and proteins can undergo various degradation in mildly acidic conditions.1 This can translate to degradation on-column when acidic mobile phases and high column temperatures are employed. In addition to being susceptible to hydrolysis reactions, peptides and proteins can become oxidized at cysteine and methionine residues2,3 It has been found that the relative abundances of peptides with methionine residues can decrease over time as those of the oxidized species increase.3 With respect to small molecules, observations of on-column degradation have increased in the last two decades, perhaps due to the ability to use a wider range of mobile phases and pH in conjunction with newer pH-resistant stationary phases.4 The use of high pH mobile phases have been especially important for the method development of basic compounds. However, these conditions have been confirmed to exacerbate oxidation.5,6 In 2011, Wang and co-authors found that on-column degradation of anilines occurred with the use of conventional metal column technology and ammonium hydroxide high pH mobile phases.5 The observed oxidation caused the formation of both azo and hydrazo dimers, which amplified over time as the column continued to be used.5
Though Wang and co-authors did not expound upon the source and cause of the oxidation, Myers and co-authors followed up on this study in 2013.6 Similar to Wang, they also saw an increase in oxidative nitrosation of their amine compounds over time. Through experimentation, they suggested that the cause was due to the reaction of ammonia forming the nitrosating agent nitric oxide. This is thought to be a metal-catalyzed reaction that may also be aggravated by the use of certain organic solvents, like acetonitrile.6,7
In this application note, we applied the insights published by Myers, Wang, and co-workers to investigate if MaxPeak Premier Technology might mitigate on-column reactions. Here, we compared the separation of a small molecule amine, the antipsychotic drug clozapine, on conventional stainless-steel columns versus ACQUITY Premier constructed with MaxPeak High Performance Surfaces (HPS). This surface has been previously shown to act as a barrier to reduce metal-analyte interactions that cause analyte adsorption.8 In this work, we observed the on-column oxidative formation of clozapine N-oxide and nitroso impurities, and noted that these species continued to grow in abundance over time when using stainless-steel columns and ammonium hydroxide modified water with acetonitrile eluent. By using a ACQUITY Premier with MaxPeak High Performance Surfaces, on-column oxidation of clozapine was found to be significantly minimized, with no impurities observed in the UV chromatograms after multiple injections.
Clozapine was prepared in 0.1% (w/v) 20/80/0.08 (acetonitrile/water/acetic acid) to a concentration of 6 mg/mL and was injected at a sample volume of 0.25 µL (0.15 µg mass load).
|
LC system: |
ACQUITY UPLC I-Class* |
|
Detection: |
UV detection at 290 nm |
|
Vials: |
Max Recovery Vials (p/n: 186002802) |
|
Column(s): |
ACQUITY UPLC BEH C18, 130 Å, 1.7 µm, 2.1 x 50 mm (p/n: 186002350) ACQUITY Premier BEH C18, 130 Å, 1.7 µm, 2.1 x 50 mm (p/n: 186009452) |
|
Column temp.: |
30 °C |
|
Sample temp.: |
4 °C |
|
Injection volume: |
0.25 µL |
|
Flow rate: |
0.31 mL/min |
|
Mobile phase A: |
0.05% (w/v) ammonium hydroxide in water |
|
Mobile phase B: |
Acetonitrile |
|
Sample diluent: |
20/80/0.08 (w/v) acetonitrile/water/acetic acid |
|
Gradient: |
25 to 80% B in 10.31 min |
|
*Use of the ACQUITY Premier System should be considered for additional protection against analyte oxidation. |
|
MS system: |
Vion IMS QTof |
|
Ionization mode: |
ESI positive, sensitivity |
|
Acquisition range: |
50–1500 m/z |
|
Capillary voltage: |
3.5 kV |
|
Sampling cone: |
40 V |
|
Source offset: |
80 V |
|
Source temp.: |
100 °C |
|
Desolvation temp.: |
250 °C |
|
Desolvation gas: |
600 L/h |
|
Quadrupole option: |
Automatic |
|
Chromatography and MS software: |
UNIFI v1.8 |
The relative amount of oxidized species (%) was estimated from the observed peak areas (UV or TIC) of clozapine and its degradation artifacts.
For this work, we aimed to study clozapine, which is an amino compound designed to treat schizophrenia. Myers and co-workers briefly discussed this compound in their paper when describing a set of amines that can undergo on-column nitrosation. Another major oxidative pathway in literature that has been known to occur is the oxidation of the tertiary amine of clozapine to its N-oxide form.9,10 Figure 1 shows the structure of clozapine and its potential degradation artifacts – clozapine N-oxide and its Z and E nitroso isomer impurities.
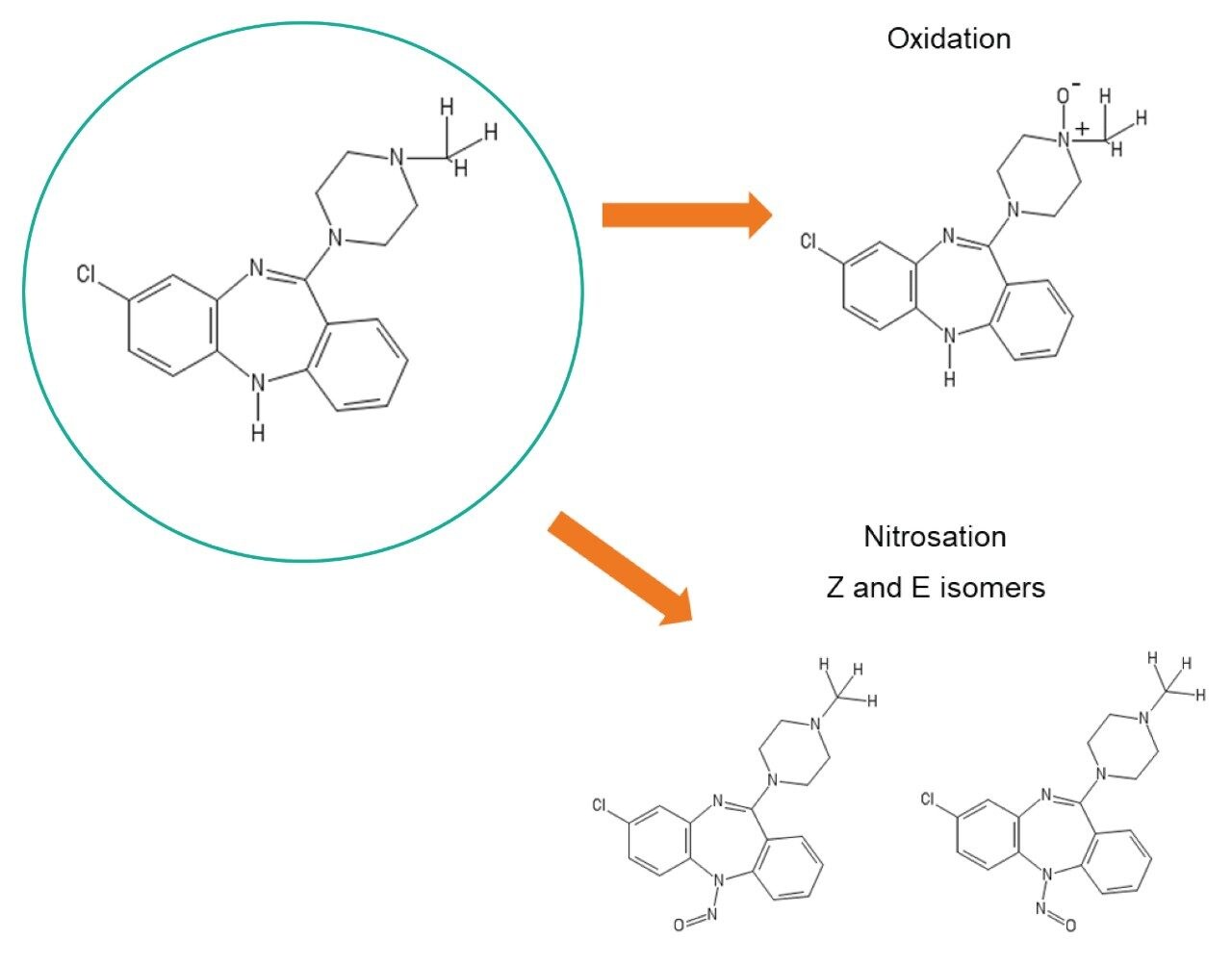
Chromatographic conditions like those described by Myers and co-authors were employed in this investigation in order to compare the production of oxidized species from the two aforementioned column technologies. Separations using 0.05% (w/v) ammonium hydroxide in water and acetonitrile as mobile phases were performed with a new and unused conventional, stainless-steel ACQUITY UPLC BEH C18, 1.7 µm, 2.1 x 50 mm Column or a new and unused ACQUITY Premier BEH C18, 1.7 µm, 2.1 x 50 mm Column. The formation of oxidized clozapine species over time were determined by monitoring UV or TIC peak areas and verified via mass analysis by MS. The experiment was run on an ACQUITY UPLC I-Class System coupled to a Vion IMS QTof.
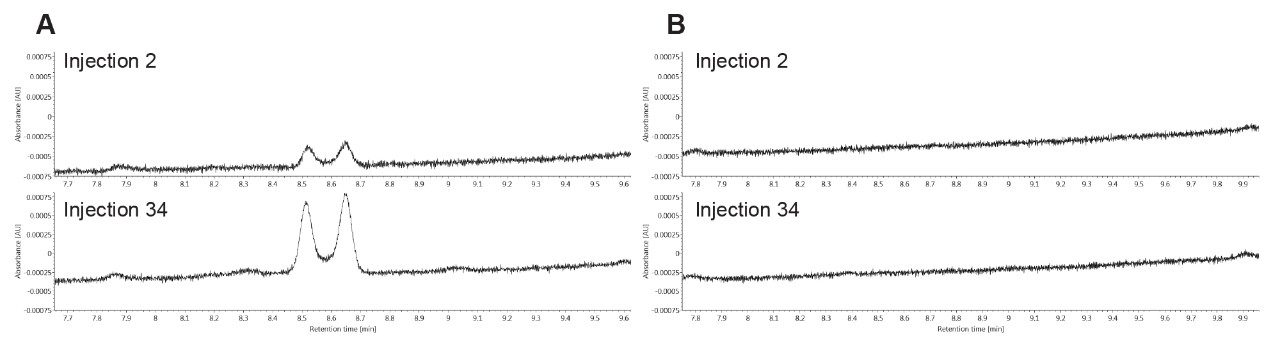
A consecutive number of injections (34) were run on both the stainless-steel ACQUITY UPLC BEH C18 Column and the ACQUITY Premier BEH C18 Column. The percentage of oxidized clozapine as correlated to UV peak area was monitored over time for each column. Figure 2 displays the UV chromatograms from the second and thirteenth injection of clozapine on the conventional steel column. Here, it can be clearly seen that there is an increase in the formation of clozapine N-oxide over time.
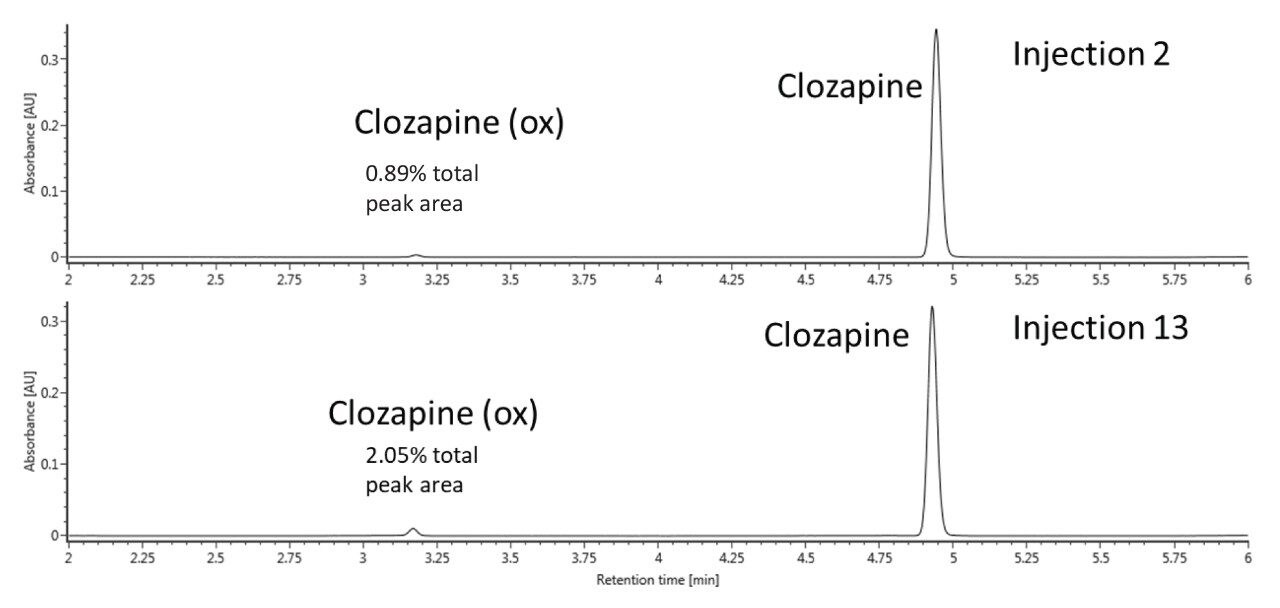
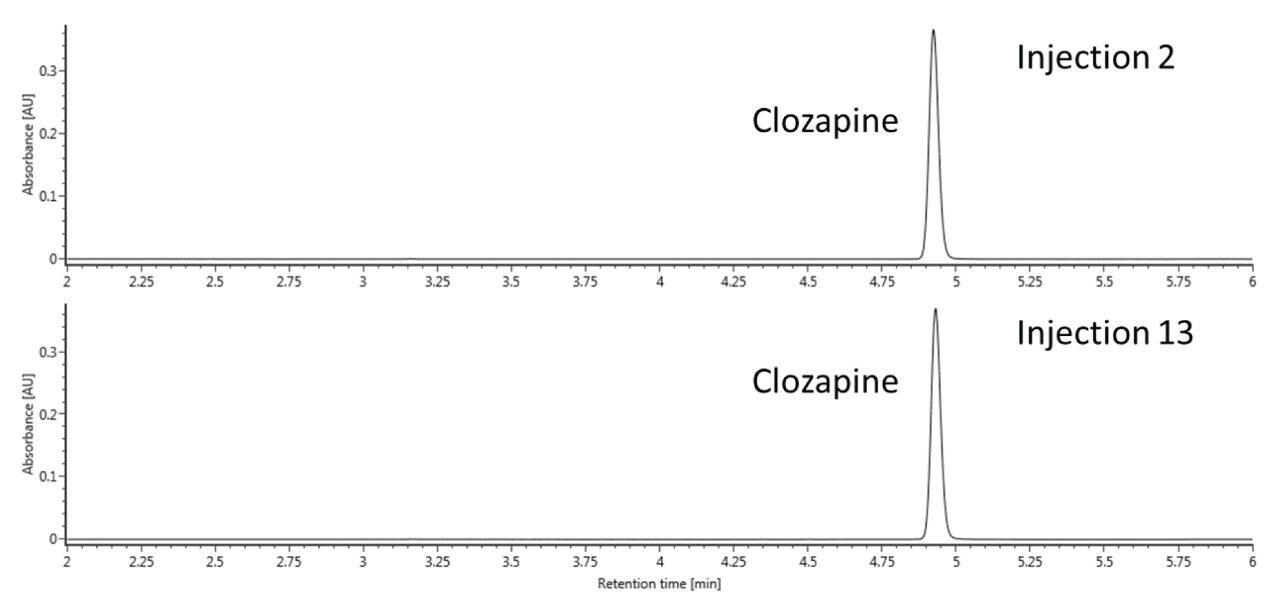
From injections 2 to 13, over a two-fold increase in the oxidation of the tertiary amine occurred, which reflects the findings in the previously cited literature. Interestingly, our results indicate that clozapine N-oxide, rather than the impurities caused by the nitrosation of the secondary amine, was the primary oxidation artifact. This appears to be in contrast to the paper published by Myers and co-authors, where the authors primarily discussed the formation of impurities caused by nitrosation over time.6 This could be due to differences in system configurations or a combination of variables; more investigation will be required to explore the differences between these studies. We also evaluated the ACQUITY Premier BEH C18 Column using the same conditions and number of injections. The UV chromatograms from injections 2 and 13 for this column are shown in Figure 3. In contrast to the stainless-steel ACQUITY UPLC BEH C18 Column, the N-oxide degradation product was not observed in the UV chromatograms on the ACQUITY Premier BEH C18 Column. These results suggest that the ACQUITY Premier Column, consisting of the hybrid organic/inorganic technology of MaxPeak HPS, can provide a barrier to the metal-analyte interactions that might cause on-column reactions, such as oxidation. This oxidation may be caused by surface corrosion on metal surfaces, especially from the frit.6 In this case, the barrier formed by the MaxPeak HPS Technology appears to protect the oxidation susceptible amine by impeding direct catalytic interactions. It might also be that the MaxPeak HPS Technology confers some degree of corrosion resistance to the metal surfaces themselves, which is of additional benefit to ensuring an accurate analysis of clozapine.
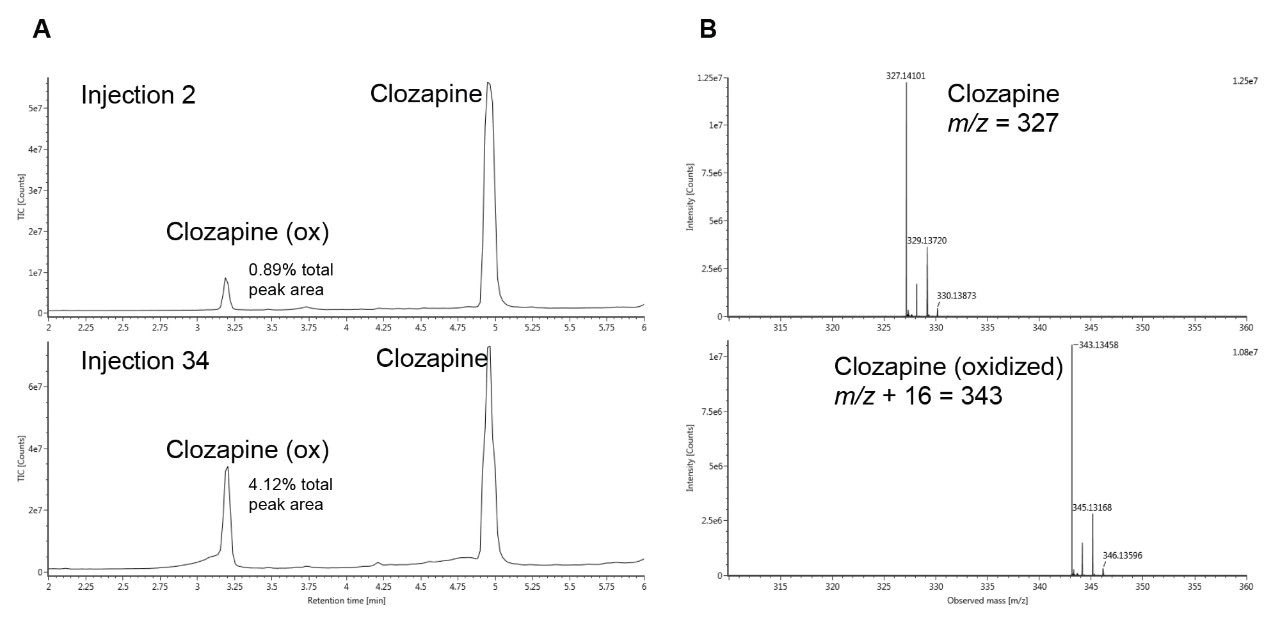
To ensure correct identification of this impurity peak, we used mass analysis to confirm the N-oxide species. The mass spectra collected from the separation of clozapine on the stainless-steel ACQUITY UPLC BEH C18 Column are shown in Figure 4B. Figure 4A shows the TIC chromatograms from the 2nd versus 34th injection, where the percentage of clozapine N-oxide has risen to 4.12% of the total peak area.
Though the primary on-column degradation of clozapine was N-oxidation, trace levels of nitrosation was also observed in the form of increasing levels of Z and E isomers. These species were found at lower levels than clozapine N-oxide and only on the stainless-steel column. Analogous to the increased formation of clozapine N-oxide over time, the nitroso species also increased between injections 2 to 34 (Figure 5). Again, these degradation products were only observed using the stainless-steel column, as the Z and E nitroso isomers were not seen on the ACQUITY Premier Column, even at injection 34.
It is interesting to note that the percent of N-oxidation throughout 34 successive injections produced an increasing and fairly linear relationship with the number of injections over time. By modelling this linear relationship, it could be suggested that significantly more N-oxide artifact might come to be found with the continued use of the stainless-steel column. To properly evaluate this relationship, an empirical study would be necessary. In addition, the formation of nitroso impurities would need to be accounted for as well.
This application note demonstrates that an ACQUITY Premier BEH C18 Column constructed with MaxPeak High Performance Surfaces is capable of mitigating on-column degradation of clozapine. The unique hybrid organic/inorganic barrier of the MaxPeak HPS appears to have prevented metal-analyte interactions that cause on-column reactions in a stainless-steel column. The work described here confirms that when a stainless-steel column is used, an analyst is likely to encounter both oxidized tertiary amines and nitrosation of secondary amines, especially over consecutive injections and prolonged use. With the use of an ACQUITY Premier Column, these degradants were not observed.
These results indicate that ACQUITY Premier Columns can provide significant advantages in improving the reproducibility and reliability of chromatographic data. This could be especially promising in many fields including that of quality control, where impurity detection could be compromised by the formation of on-column degradation products. Additionally, we foresee that this technology could be applied towards mitigating on-column reactions of other analytes, such as the oxidation of amino acid residues in peptides and proteins as well as the oxidation of some types of oligonucleotides.
720007160, February 2021