
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the method transfer for a fast gradient method from an HPLC legacy system to the Arc HPLC System. The quaternary Arc HPLC System provides greater injection precision with retention time repeatability comparable to the binary HPLC systems.
With the globalization of resources, method transfer across different laboratories and geographical locations has been an area of increased interest. While many scientists prefer to transfer methods across similar systems, this is not always possible. While the different pumps, quaternary vs. binary, can impact retention time characteristics, other aspects, including injection precision, are a characteristic of the sample manager. Regardless of the system, both injection and retention time precision are important performance characteristics of any method. In this example, we have demonstrated the transferability and effect on key assay performance characteristics of a fast gradient method from a legacy binary HPLC system to an Arc HPLC System.
Method transfer across binary and quaternary systems present unique challenges because of differences in gradient delay or dwell volumes, gradient delivery mechanism, and mixing across the two types of solvent mangers (high pressure vs. low pressure). While some methods are better suited for one type of pump over the other,1 many routine HPLC methods will meet system suitability criteria on either system. Specifically, these are methods that are within the performance specifications of both types of pumps, including starting %A and B, and flow rates. Under these conditions, methods are typically transferable and may only require adjustments for delay volume if absolute retention time criteria are used.
In this example, a very fast gradient separation of a small molecule mixture was transferred from a legacy binary HPLC system to the Waters Arc HPLC System. Methods with this very rapid gradient are susceptible to changes in mixing modes and gradient delay volume. Comparison of the separation shows a shift in retention time between the two systems (Figure 1 and Table 1). This shift corresponds to the difference in the gradient delay volumes of these two specific systems, or roughly 0.200 mL. While absolute retention time may be a critical aspect of a separation, relative retention times are commonly used to reduce the impact of the delay volume on peak confirmation or identification. In this case, comparison of the relative retention time, using valerophenone as a reference, demonstrates the same selectivity across the two methods.
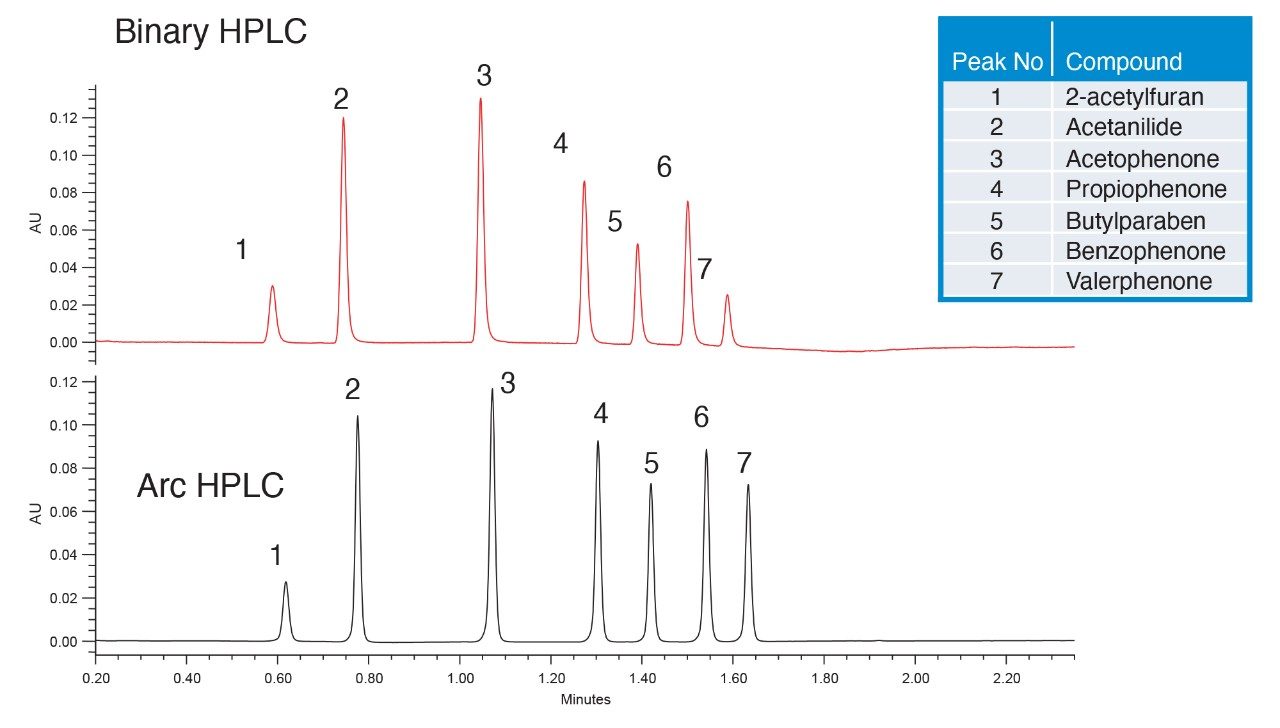
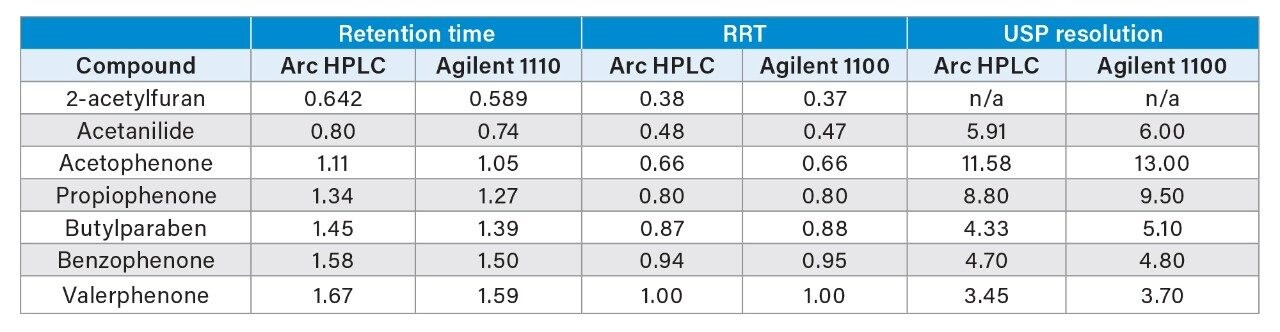
Further confirmation of the separation fidelity is provided by other chromatographic properties, including USP resolution of the two separations (Figure 1 and Table 1). While there was slight variability of some USP resolution values, all the peaks were well separated with USP resolution >2.0. Both systems also produced USP tailing of 1.0-1.3, within accepted values. Slight system differences (e.g., fittings and preheaters) could potentially contribute to some variability. In addition, the two analyses were performed at different time points, with two different, specific columns and sample preparations. As a result, column-to-column variability cannot be ruled out as slightly impacting basic chromatographic performance characteristics.
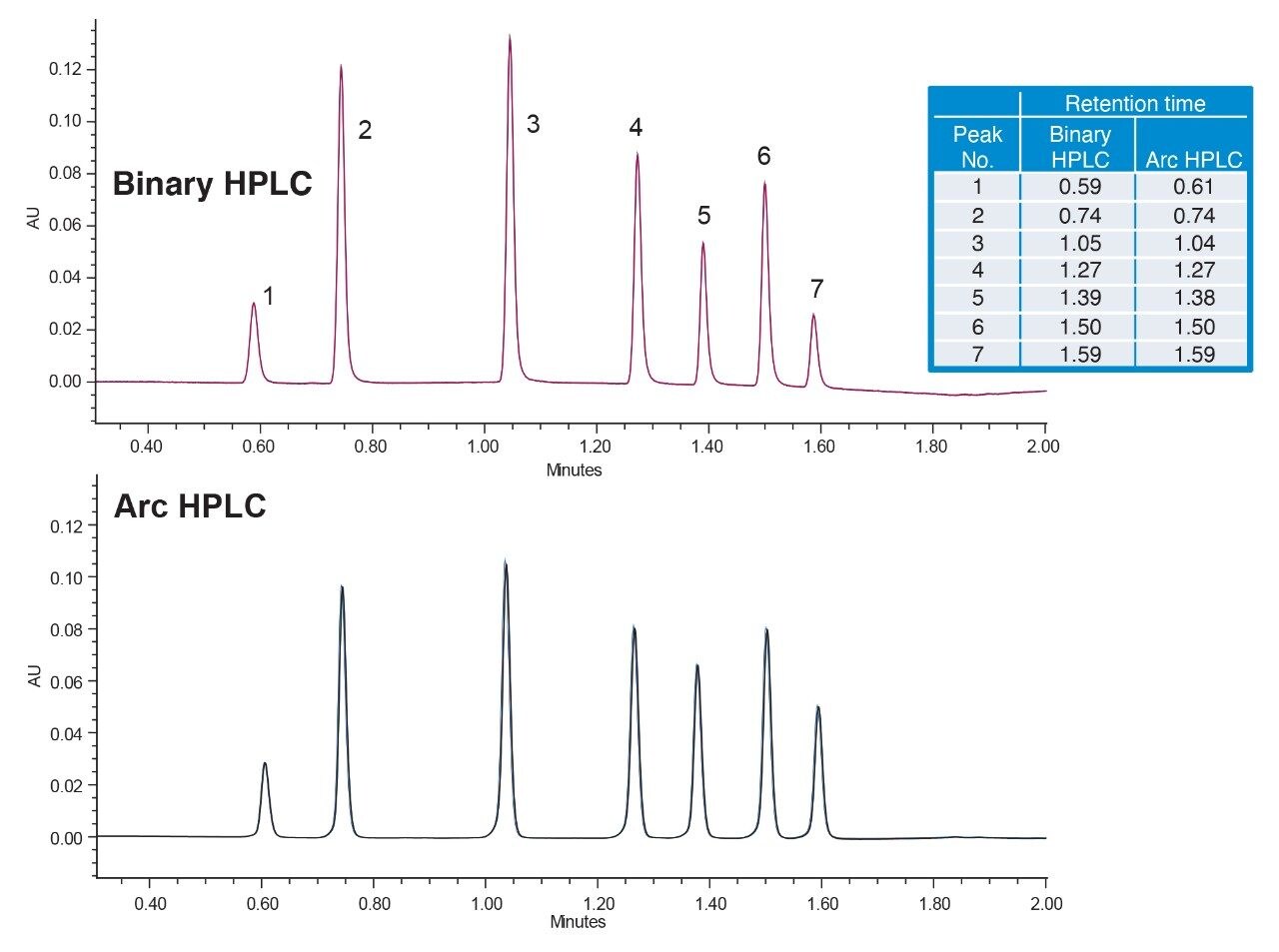
Given the differences across systems in the gradient delay volume, adjustments for gradient delay are generally permitted in method transfer.2 To ensure the same separation is obtained on both systems, Gradient SmartStart was used to adjust the gradient start relative to the injection without modifications to the gradient table.3 Based on the results, adjustments were made for the gradient to start 0.05 min before injection. This resulted in lower delay volume on the Arc HPLC System and absolute retention times more closely matching the binary HPLC system, eliminating the need for relative retention times (RRT) requirements (Figure 2).
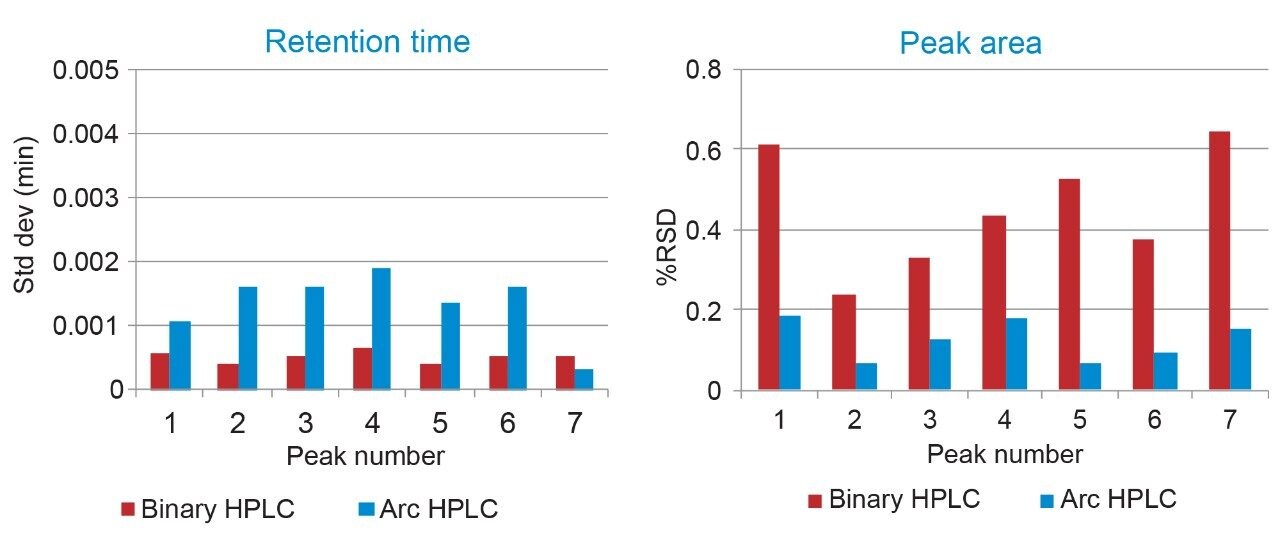
To assess the precision of both the pump and the sample manager, six replicate injections were performed on each system. As described earlier, retention time precision is a function of the mobile phase delivery system, while area or injection precision is impacted by performance of the sample manager. Comparing the two systems, we can see low retention time standard deviations for each system (<0.002 min or 7 µL), well within typical specifications for such a fast method, indicating reproducible mobile phase delivery, regardless of pump type. The peak area %RSDs demonstrate the high precision of the Arc HPLC System sample manager at injection volume of 20 µL with values of <0.2% (Figure 3).
For many typical HPLC methods, transfer from a binary system to a quaternary can produce comparable results. Specifically, the transfer of a challenging, very fast gradient separation on a 4.6 x 50 mm column demonstrated similar retention time and resolution results. Small differences in absolute retention time were observed due to gradient delay of each pump, requiring an evaluation of the relative retention times. By adjusting the gradient start relative to the injection, with Gradient SmartStart, comparable absolute retention times were observed on both systems. High retention time precision was observed on both pumps with standard deviations of less than one second or 7 µL, while the injection precision on the Arc HPLC System was superior to the legacy binary LC system.
720006943, June 2020
Upgrading HPLC: Transferring Fast Gradient Methods to Arc System
Method transfer stands as a pivotal practice within chromatography, especially when migrating from one High-Performance Liquid Chromatography (HPLC) system to another. In this context, we delve into the intricacies of transferring a fast gradient method from a binary HPLC system to an Arc HPLC system. The binary HPLC system deploys two solvents in a predefined ratio for its mobile phase, suitable for routine analytical tasks. Contrarily, the Arc HPLC system is quaternary, offering the flexibility to use up to four different solvents. It excels in managing complex separations and facilitates method development.
The necessity for method transfer emerges when upgrading or changing HPLC systems. Variations in instrument design, flow pathways, and pump configurations can significantly influence the chromatographic outcomes, making method transfer paramount. The transition in this instance entails moving from a more basic binary system to the versatile Arc HPLC system.
Comprehensive Breakdown of the Method Transfer Process
Method transfer from a binary HPLC to an Arc HPLC system involves a systematic ten-step process. It commences with evaluating the existing binary HPLC method's specifics, such as column choice, mobile phase composition, and gradient parameters. Subsequent steps include selecting an appropriate column for the Arc system, optimizing the mobile phase, recreating the gradient to match the Arc's flexibility, transferring parameters related to injections and detection, conducting an initial method test, fine-tuning based on results, validating with established samples, extensive method documentation, and routine maintenance and system suitability tests. This process ensures the successful adaptation of the method to the new system while maintaining accuracy and reliability.
The benefits of adopting the Arc HPLC system in this method transfer are substantial. This system provides the capability to finely adjust gradients by utilizing a wider array of solvents. Moreover, it enhances injection precision, ensures better retention time repeatability, and promotes improved peak shapes. These advantages prove especially valuable when dealing with fast gradients and more intricate separations, making the Arc system an indispensable tool for achieving heightened method flexibility and analytical precision.
In sum, method transfer from a binary HPLC system to an Arc HPLC system constitutes a meticulous process of optimizing method parameters to ensure optimal functionality within the new system. This methodological shift encompasses a comprehensive evaluation of the initial method, the recalibration of mobile phase and gradients, and the validation of results to guarantee reliable and replicable chromatography on the Arc system. Embracing the Arc HPLC system empowers chromatographers with a versatile and advanced tool that elevates precision and flexibility in analytical procedures.