
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates how method conditions and instrument settings can be optimized for the detection of free fatty acids in LC-MS-based analyses.
Structural considerations play a critical role during method development in the detection and identification of free fatty acids in LC-MS-based analyses.
Free fatty acid (FFA) analysis occurs in a variety of research fields including pharmaceutical, food and environmental, proteomics, biopharmaceutical, and material sciences. Physicochemical properties of FFAs such as chain length and degree of saturation can impact the separation efficiency and detector response of a given technique. Gas Chromatography (GC) is one of the more popular analysis techniques of fatty acids, however derivatization of the fatty acid is often required to increase instrument compatibility. MS-based methods offer the means to avoid costly and time-intensive derivatization steps through the direct analysis of FFAs. Positive ion detection modes typically employed with conventional RPLC-MS mobile phases arenot ideal in the detection of FFAs as loss of water can readily occur, thus impacting assay sensitivity and data interpretation. As LC-MS-based methods are increasingly deployed in the lab setting, the need to improve MS response becomes more evident, specifically methods that are optimized for FFA analysis in negative ion mode.
Structural considerations of analytes under investigation play a critical role in the selection of mobile phase conditions and instrument settings in LC-MS-based analyses. Reversed-phase chromatography is well suited in the analysis of FFAs when considering their hydrophobic properties, however conventional RPLC mobile phases using acidic modifiers tend to induce water loss in the MS source when acquiring data in positive mode as the carboxylic group can readily accept protons. Given their structural properties, MS acquisition in negative mode is fundamentally more appropriate for fatty acids analysis as water loss is not thermodynamically favorable. To this end, a conventional RPLC-based mobile phase comprised of 75:25 MeCN:H2O, 0.1% FA, pH ≅ 3.0 with MS data acquired in positive mode was compared against a mobile phase containing 75:25 MeCN:H2O, 10 mM NH4HCO2, pH ≅ 7.0 with MS data acquired in negative mode. Lauric acid and an internal standard (IS) were prepared at 0.1% w/w and analyzed under optimized RPLC conditions using an ACQUITY UPLC BEH C8 Column (130Å, 1.7 μm, 2.1 mm x 100 mm, p/n 186002878) on an ACQUITY UPLC H-Class Bio PLUS System. Samples were run using an isocratic method with a flow rate of 0.2 mL/min at 30 °C. The ACQUITY QDa Mass Detector was configured in-line to evaluate spectral response. As shown in Figure 1, chromatographic performance of lauric acid when using 0.1% FA was acceptable with minimal tailing and a resolution of 5.63 from the internal standard. However, as shown in the MS spectra, peak splitting due to water loss was observed at approximately 50%. As shown in Figure 2, chromatographic performance of lauric acid was preserved with comparable peak shape, retention time, and a resolution of 5.39 from the internal standard when using 10 mM ammonium formate in lieu of formic acid. More importantly, spectral peak splitting was not observed at an appreciable level when acquiring MS data in negative mode as shown in the figure inset. This work demonstrates that spectral response of FFAs can be improved when considering structural properties during method development to reduce in-source water loss peaks for efficient spectral interpretation.
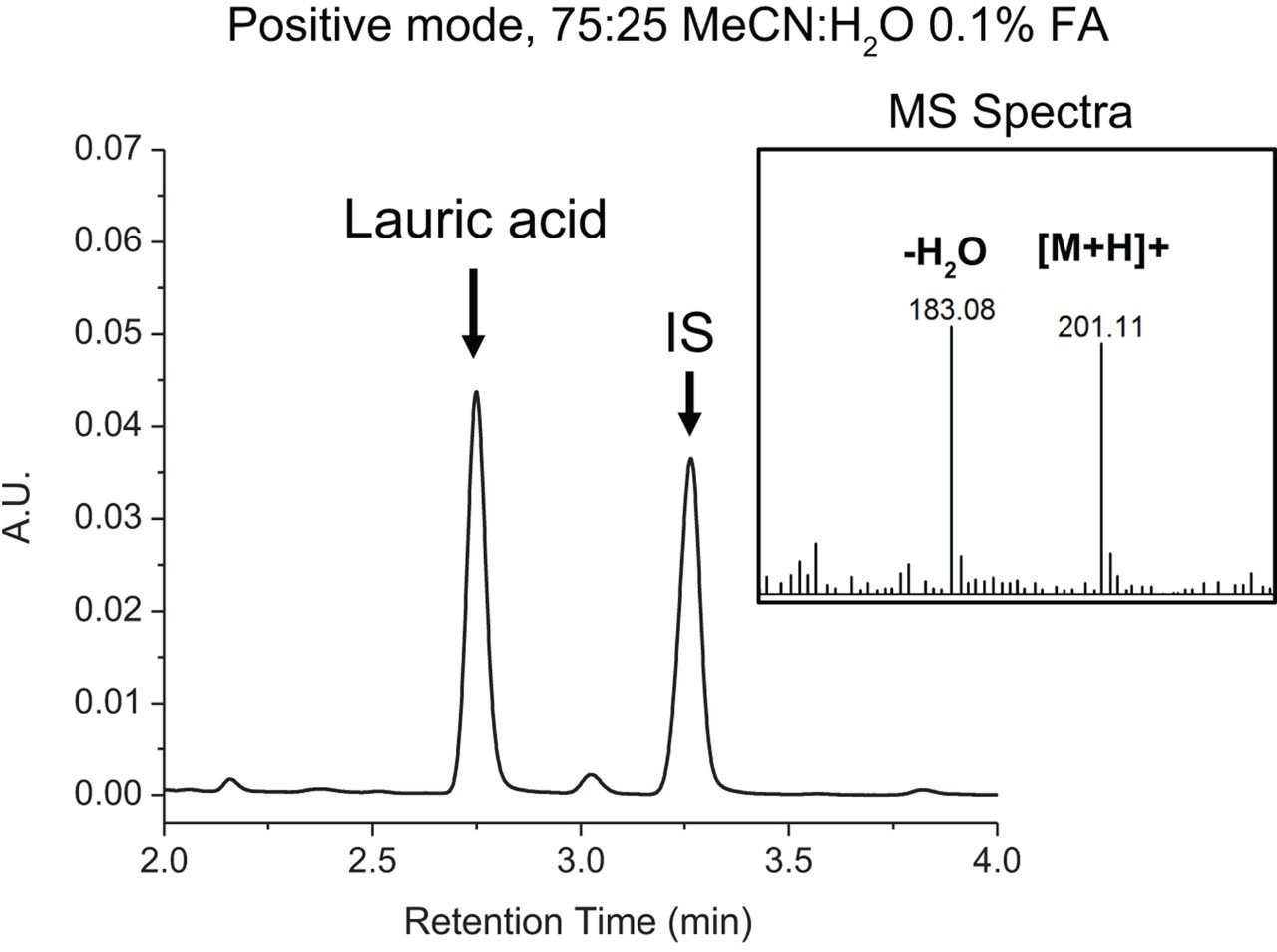
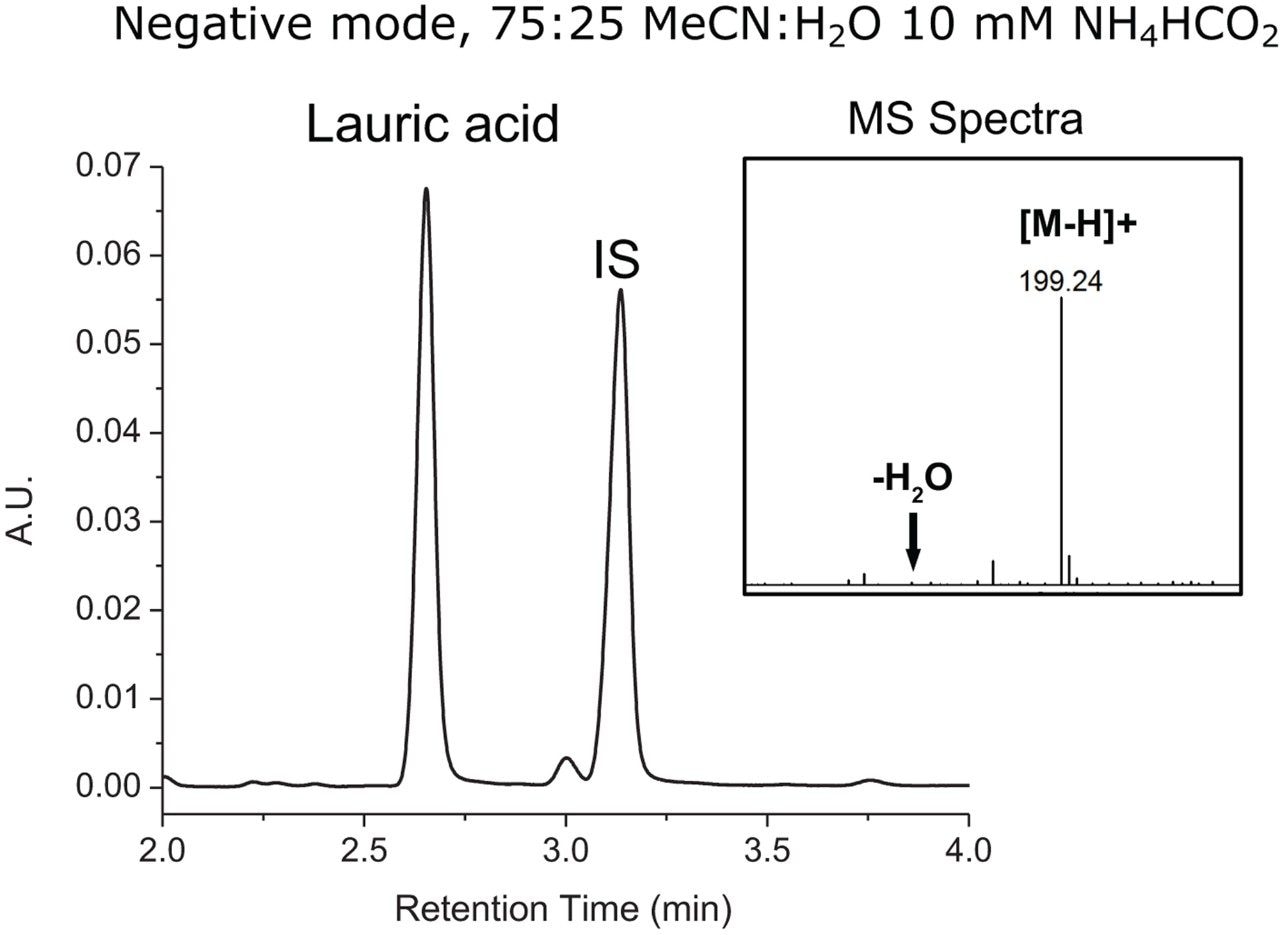
Structural consideration of target analytes at a fundamental level can be used to guide method optimization in RPLC-MS-based analyses. In this study, in-source water loss in the analysis of fatty acids was mitigated by acquiring MS data in negative mode while retaining chromatographic performance.
720006624, August 2019