In this application note, we report the potential of a new data independent acquisition (DIA) mode (SONAR) on a QTof instrument in combination with a scanning quadrupole mass filter and ultra-fast detection system. This methodology along with Vion ion mobility enabled QTof-MS (IM-QTof-MS) were used as tools to improve analytical selectivity and facilitate the process of marker identification in complex juice samples following a simple sample preparation step.
Benefits of SONAR for the application of fruit juice metabolomic profiling for quality control and authenticity purposes include:
Comprehensive identification of phytoactive compounds is a critical starting point for assessing the biological and technological properties in food matrices. Due to the complexity of plant secondary metabolism the full characterization of phytochemicals in fruits and vegetables is recognized as a significant analytical challenge and requires sensitive and accurate techniques to be employed. Pomegranate fruit (Punica granatum L.) is commonly reported as a rich dietary source of phenolic compounds with regular consumption being linked to a wide range of associated health benefits. Phenolic compounds are also known to play an important role in the quality and sensorial performance of fruit juice products and as such of value to the food industry.1
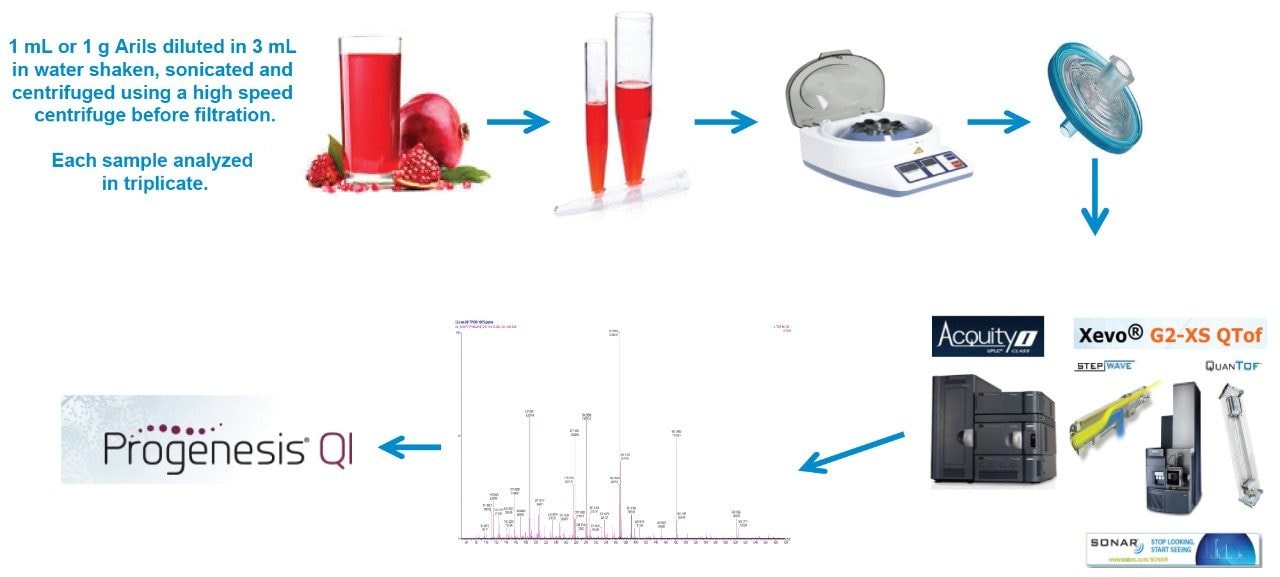
In this application note, we report the potential of a new Data Independent Acquisition (DIA) mode (SONAR) on a QTof instrument in combination with a scanning quadrupole mass filter and ultra-fast detection system.2-3 This methodology along with Vion ion mobility enabled QTof-MS (IM-QTof-MS) were used as tools to improve analytical selectivity and facilitate the process of marker identification in complex juice samples following a simple sample preparation step, as illustrated in Figure 1. The resulting information was further subjected to database searching which indicates the presence of several significant polyphenolic compounds and processing additives in a selection of commercially available processed juice products in the UK.
|
UPLC system: |
ACQUITY UPLC I-Class |
|
Column: |
ACQUITY UPLC HSS T3, 100Å, 1.8 µm, 2.1 mm × 100 mm (p/n: 186003539) |
|
Column temp.: |
45 °C |
|
Injection volume: |
3 µL |
|
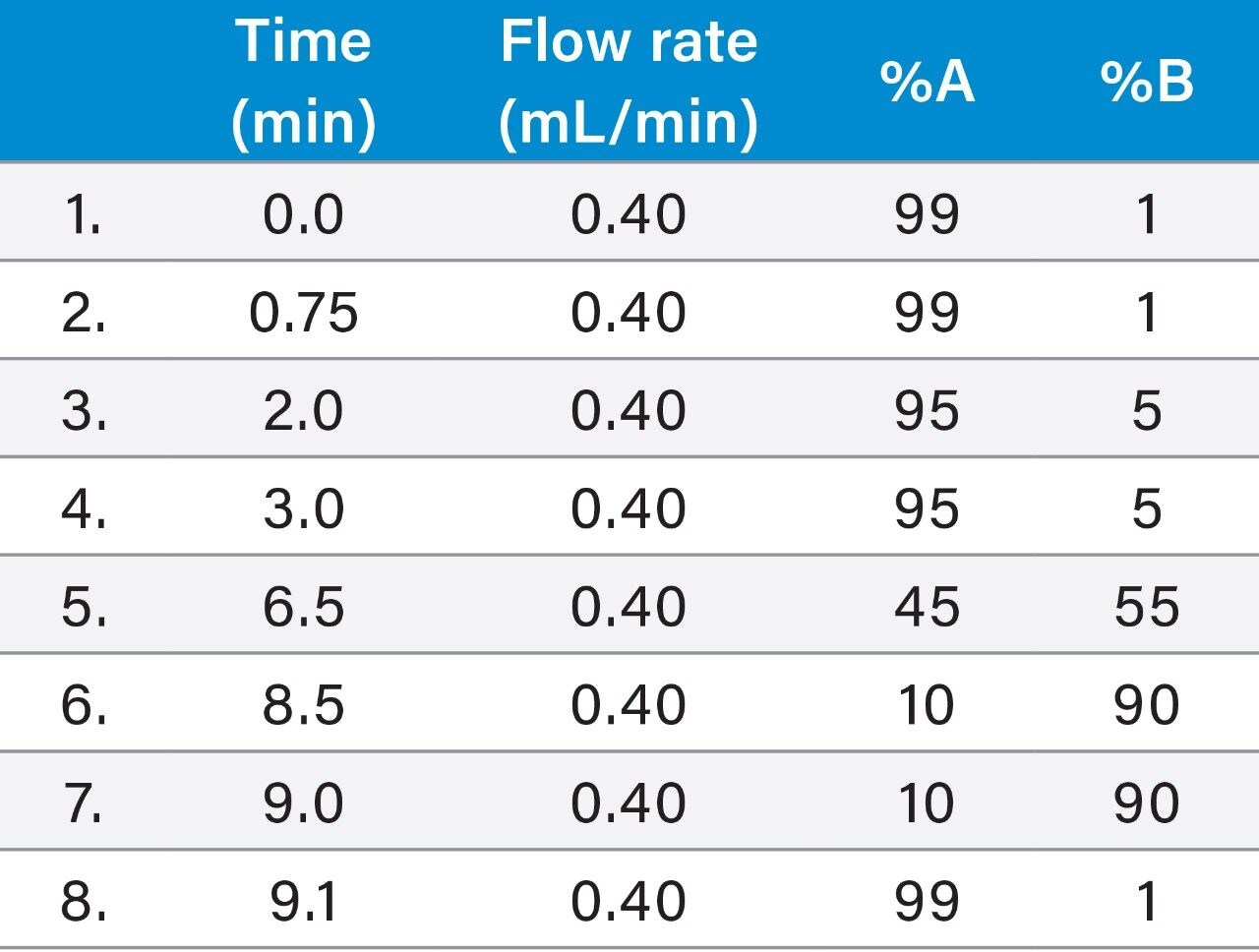
Flow rate: |
0.4 mL/min |
|
Mobile phase A: |
10 mM Ammonium acetate with water |
|
Mobile phase B: |
Acetonitrile |
|
MS system: |
Xevo G2-XS QTof, SONAR-enabled |
|
Analyzer mode: |
Sensitivity |
|
Ionization mode: |
ESI |
|
Capillary voltage: |
2.1 kV |
|
Sampling cone: |
90 eV |
|
Desolvation temp.: |
600 °C |
|
Desolvation gas: |
800 L/hr |
|
Source temp.: |
150 °C |
|
MSE Low energy CE: |
4eV |
|
MSE High energy CE: |
10–30 eV |
|
Acquisition range: |
50–1200 m/z |
|
Scan time: |
0.1 sec |
|
SONAR start mass: |
100.0 DA |
|
SONAR stop mass: |
500.0 DA |
|
SONAR quad peak width: |
20 |
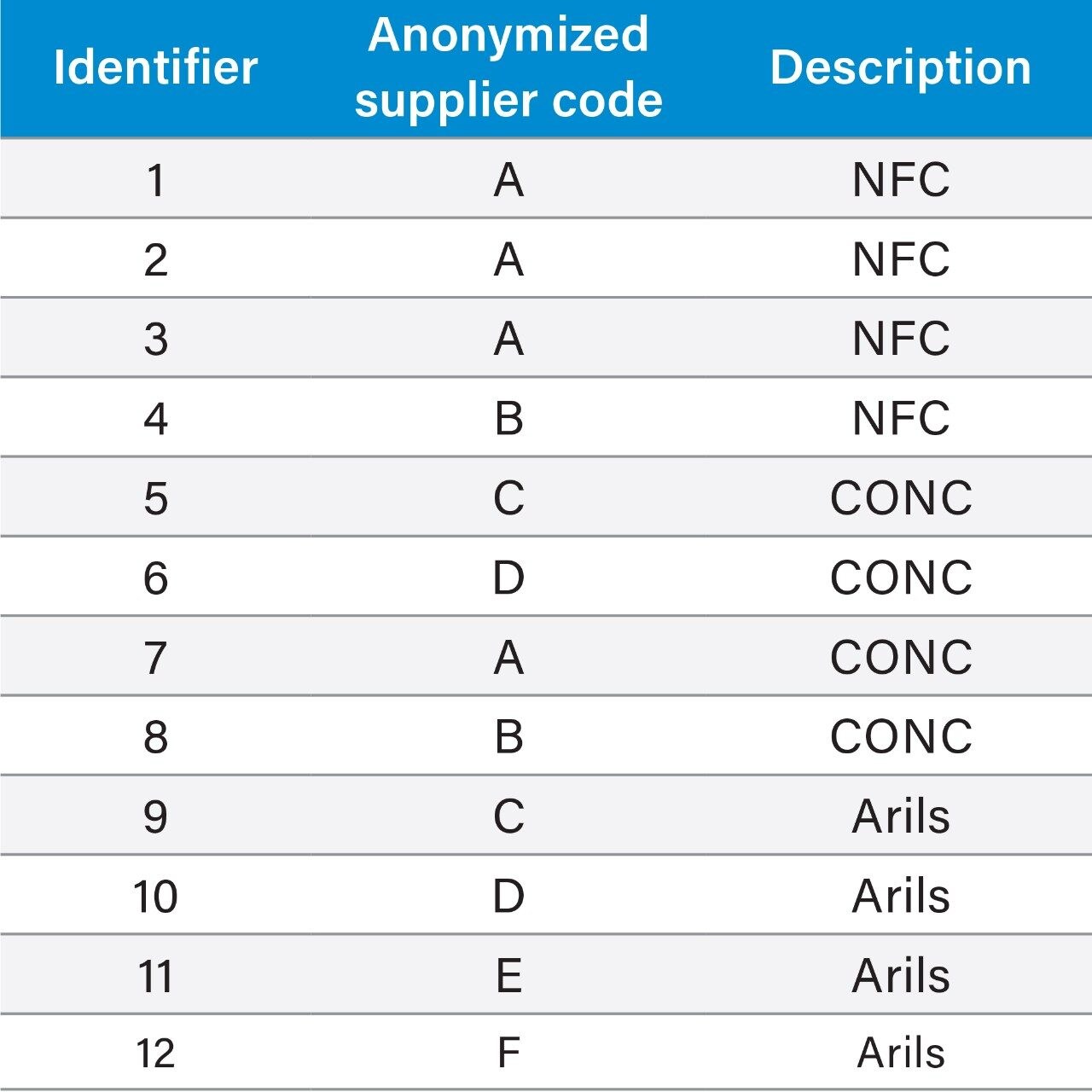
A number of retail pomegranate juices were purchased and analysed for the purpose of the study. The sample set included four different samples representing each of the following categories of fruit juice:
Pooled samples were prepared from each individual sample type (NFC, CONC, and Arils) and a global QC Pool was prepared from all sample types
1 mL of neat pomegranate juice (or 1g Arils crushed with pestle and mortar) were pipetted or weighed into a 15 mL falcon tube. 3 mL of LCMS grade water was added to the juice. The extract was centrifuged at 6000 rpm for 6 mins at room temperature. In each case, 3 mL of supernatant was removed and filtered using a Waters Acrodisc Syringe Filter, PVDF, 25 mm, 0.45 µm, Aqueous, p/n: WAT200510.
The different phytochemical fingerprints of pomegranate juice types were determined using UPLC-ESI-QTof operated in negative ionization mode with SONAR enabled acquisition.
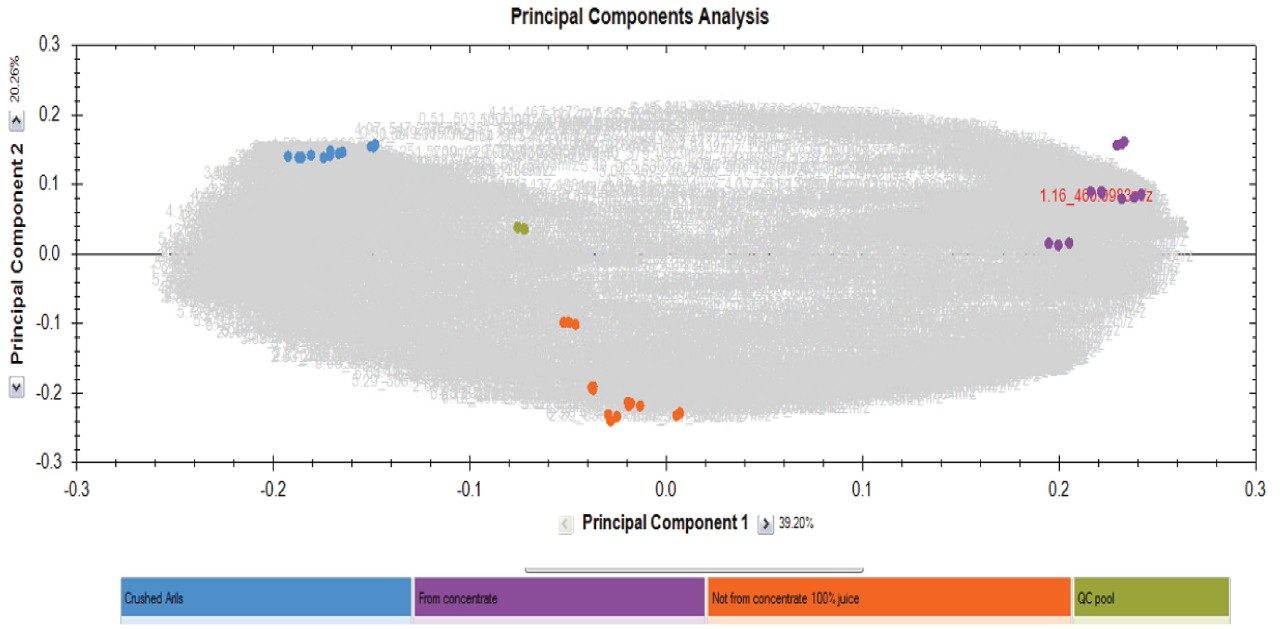
Figure 3 shows the scores and loadings plot of multivariate statistical analysis in Progenesis QI Software (PCA following univariate scaling ANOVA p≤0.05; fold change ≥2). The analysis revealed three distinct populations grouped according to the juice preparation type. These were visualized following unbiased non-targeted analysis; not from concentrate (NFC); juice/drink and fresh fruit (Arils) only with the QC pool clustering centrally.
Compound level interrogation of the data highlighted in Figure 4, shows the abundance profiles in the juice samples. Phytochemicals including members of the flavonoid-o-glycosides were found to predominate in the Aril and 100% juice samples compared to the NFC juices, whereas various permitted food dyes, flavorings and preservatives were detected in the juice/drink samples. The abundance plots reveal that the flavonoid compound, myricetin-3-galactoside to be elevated in both the freshly squeezed (Arils) and 100% juice sample sets (a) and the synthetic dye compound, C.I. Pigment red 149, to be elevated in the with CONC samples (b).
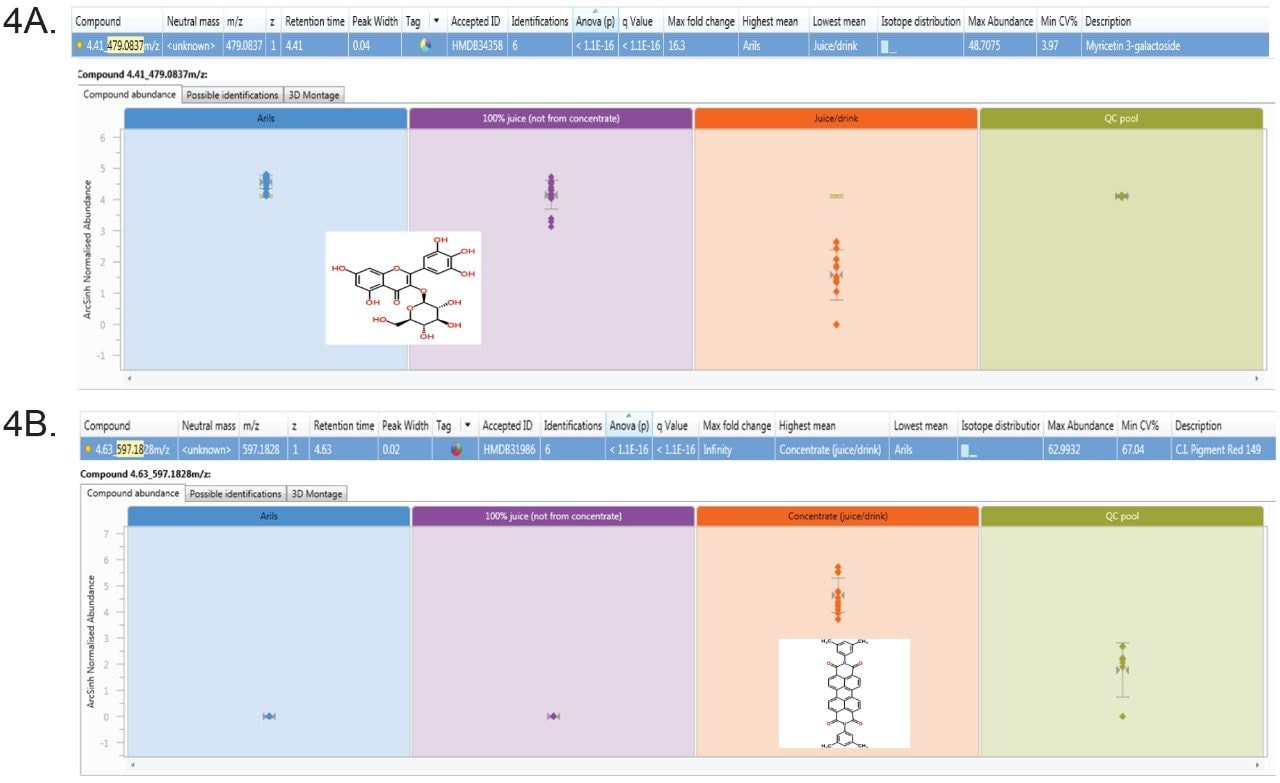
Figure 5 depicts the S-plot generated following OPLS-DA analysis of the Arils vs. CONC samples. The further statistical analysis was performed using EZinfo to identify the significant marker ions indicative of the different processed juice types. A subset of selected markers were further subject to database searches for compound identification.
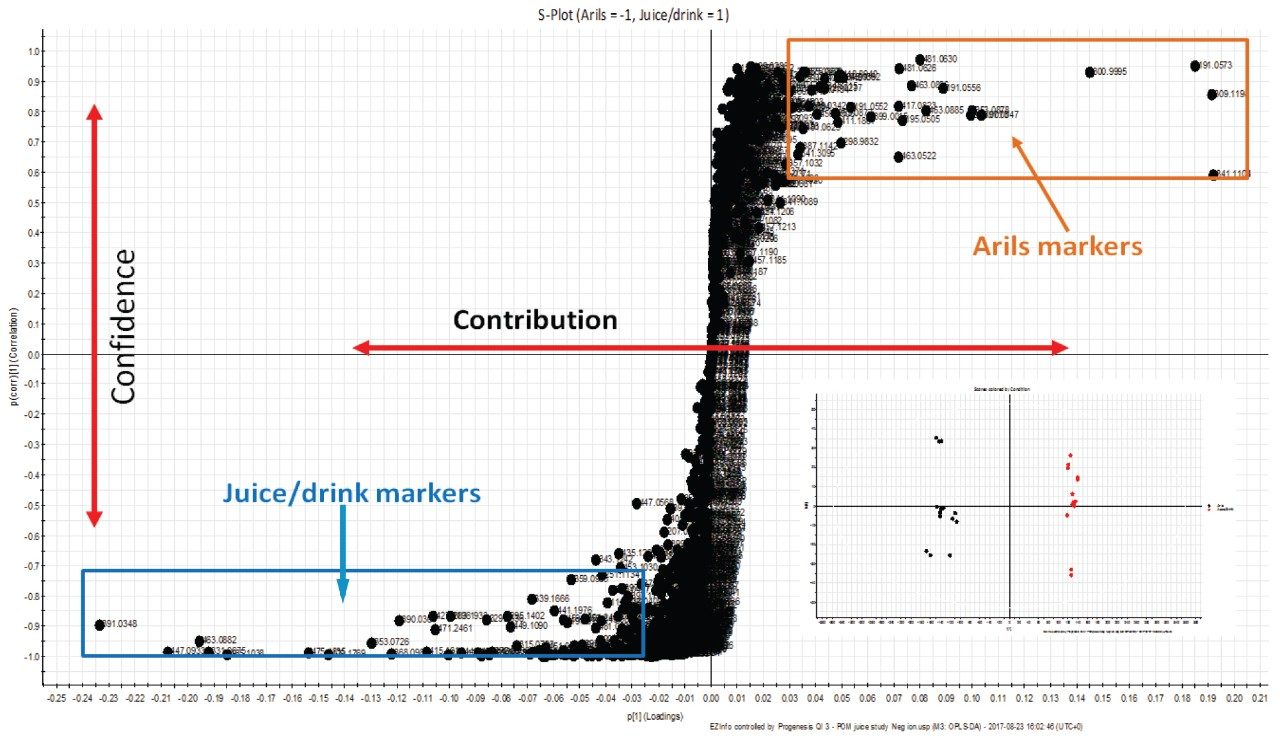
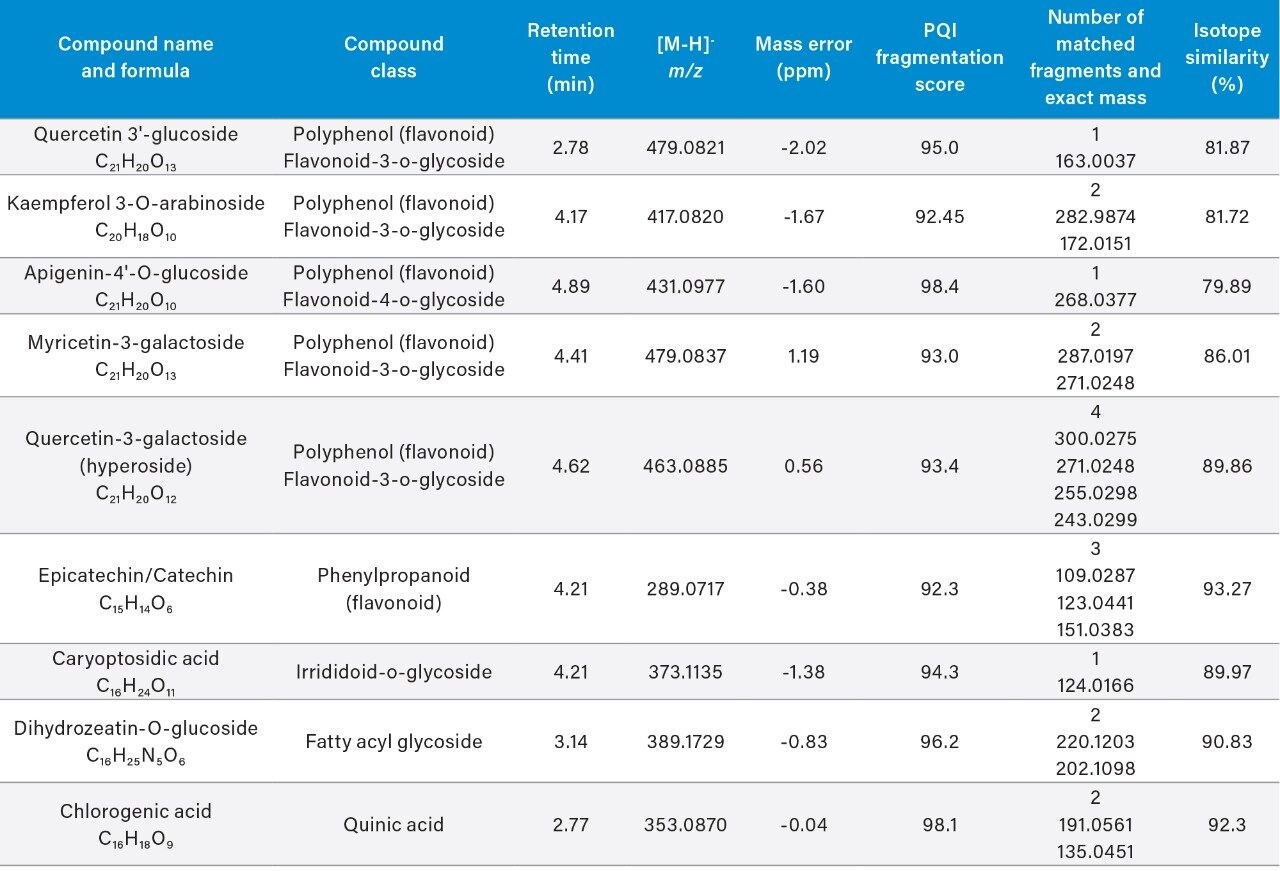
Table 3 shows the tentative chemical assignments based on precursor and fragment ion accurate mass and isotopic pattern matching against published databases (Phenol Explorer and HMDB) for 12 representative ions from the “Aril marker” subset. The assignment was conducted using the Metascope search engine in Progenesis QI.
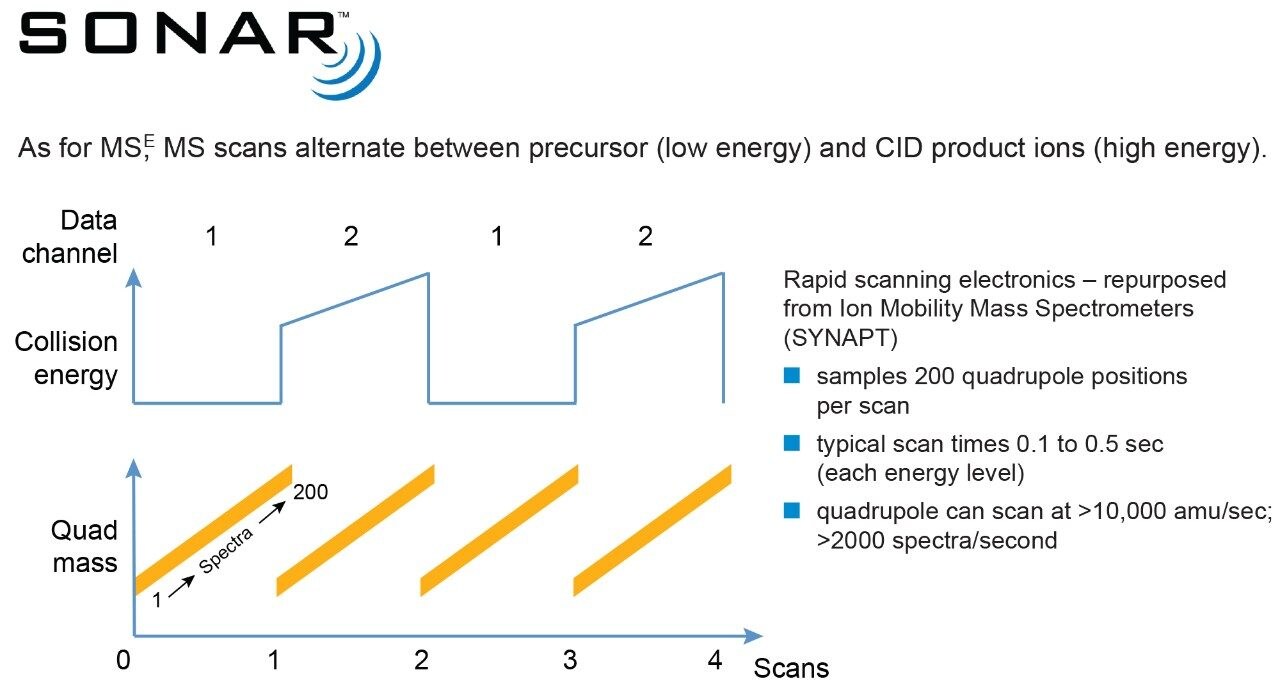
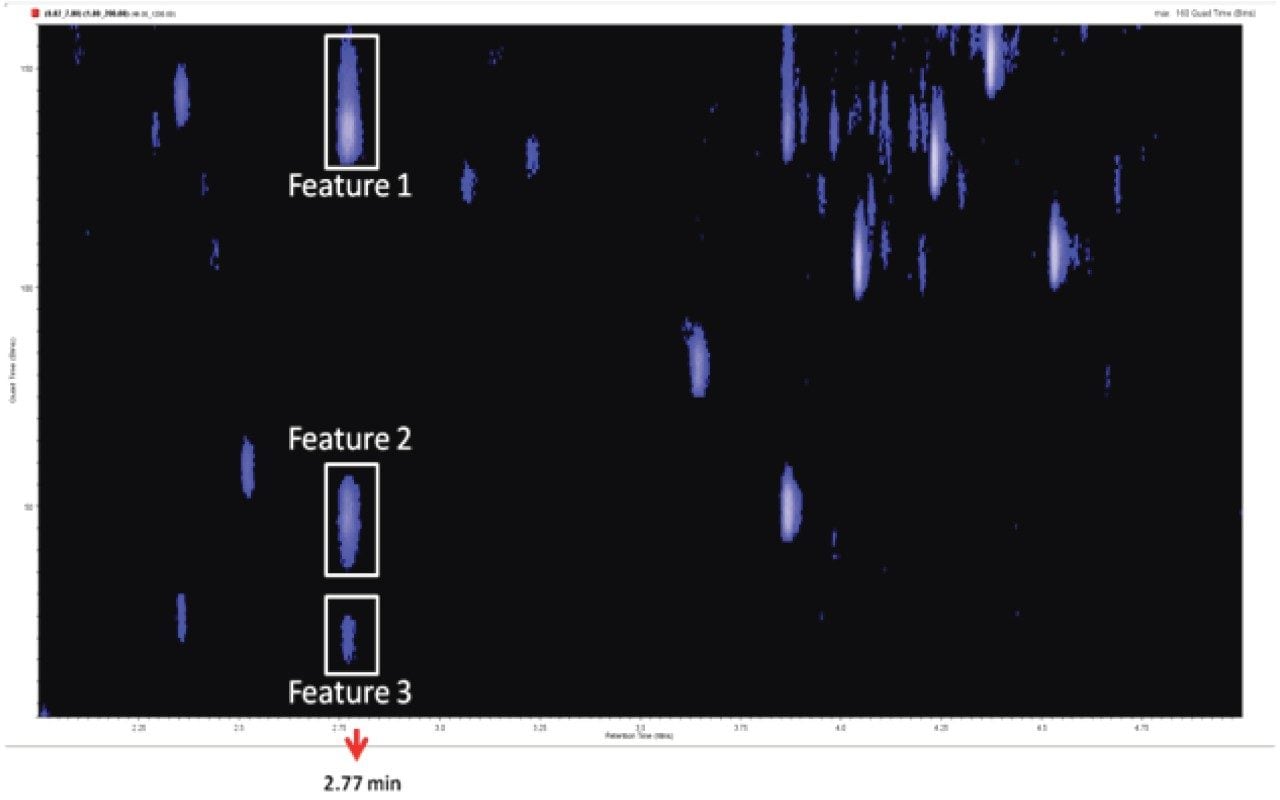
Inspection of the SONAR DIA data revealed the presence of three chromatographically co-eluting features having a retention time of 2.77 mins with different precursor masses and common fragment ions. Figure 6 shows the heat map visualization of the data plotting the quadrupole scan window (y-axis) versus chromatographic retention time (x-axis). Using conventional DIA it was not possible to determine the relative contributions of the precursor and fragment ions in the low and high energy channels. The additional selectivity provided by the resolving quadrupole in the SONAR DIA is seen to facilitate spectral interpretation allowing precursor and fragment ions to be more confidently associated.
Compounds that are chromatographically co-eluting are now separated by SONAR and recorded individually (Figure 7) making the library searching more facile and thus improving the fragmentation scores obtained using Progenesis QI compared to conventional DIA techniques such as MSE.
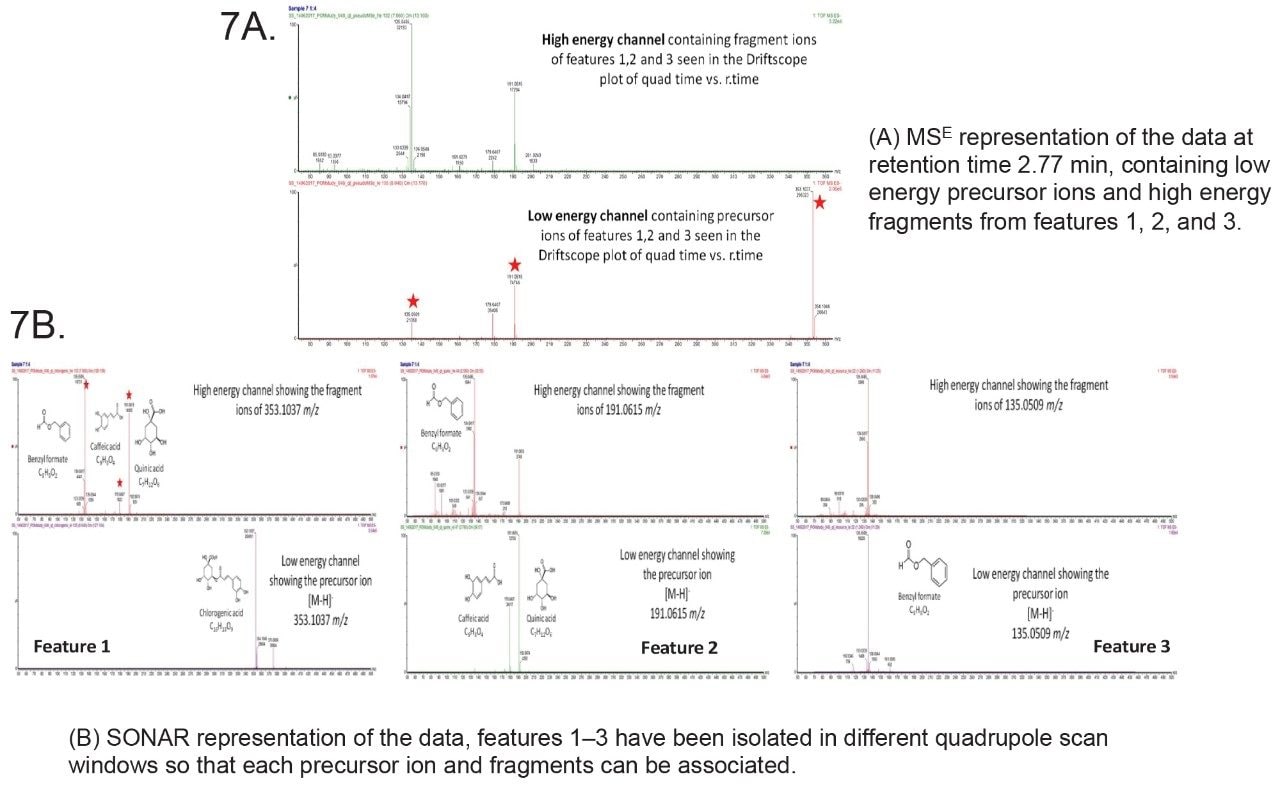
Figure 8 shows a specific example of spectral clarity on moving from traditional DIA acquisition to SONAR acquisition for the identified component quercitin-3-galactoside, a member of the flavonoid glucoside subclass.
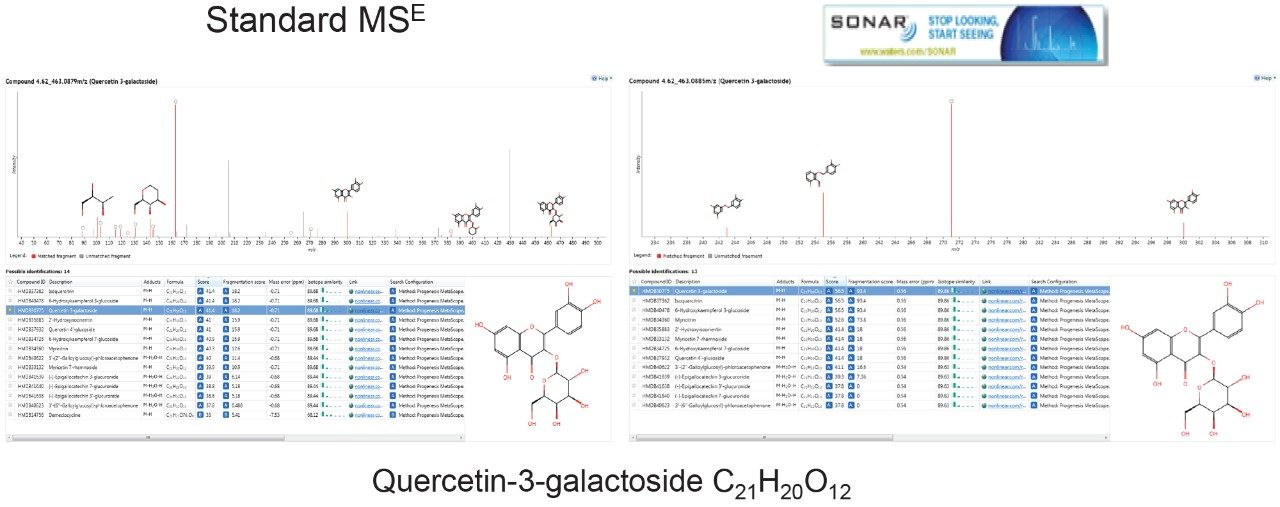
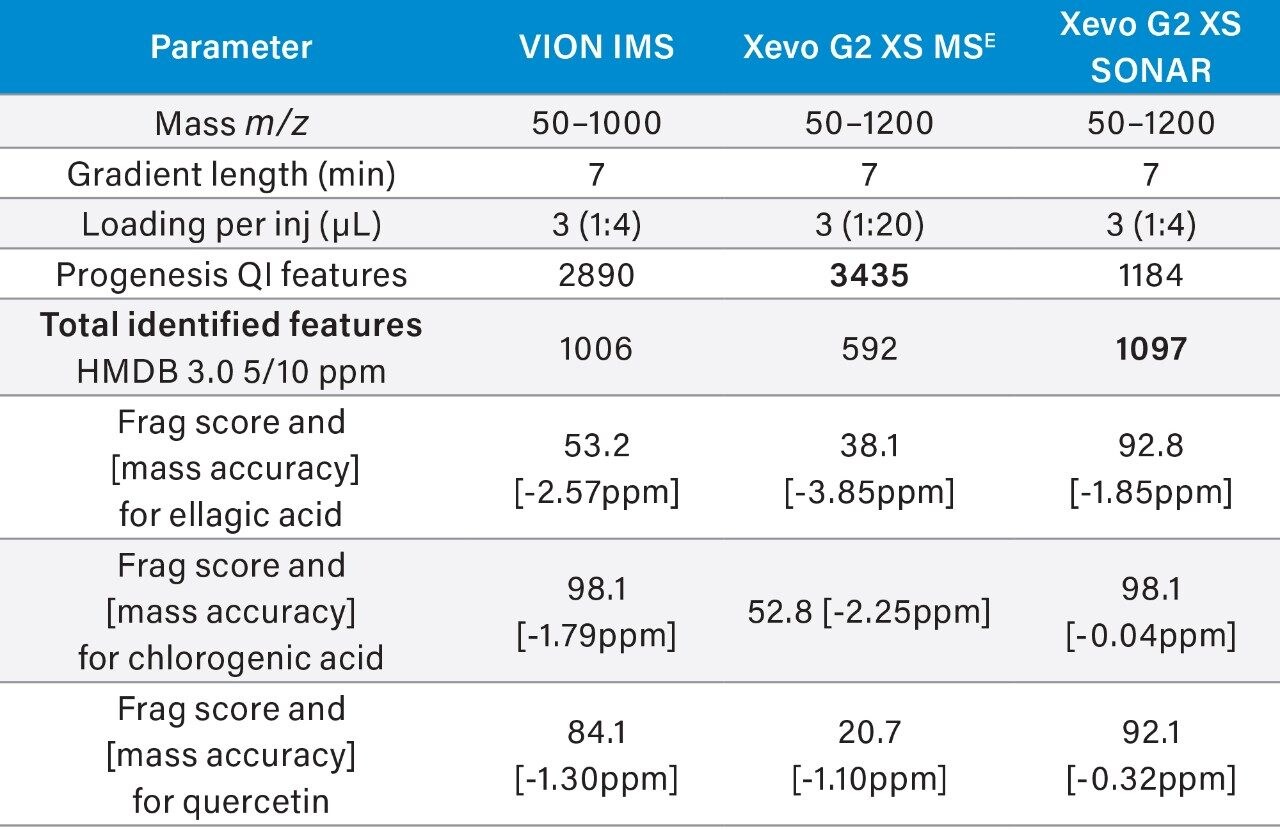
Table 4 highlights the comparative retail juice experiment performed using traditional DIA, against those which also included SONAR and IMS. Both IMS and SONAR are shown to increase the number of identified components and show increased fragmentation scores. The highest of these is shown to be as a result of SONAR analysis.
However, an additional feature of the IMS experiment is the ability to separate isobaric compounds. Isocitric/citric acid ratio is commonly used as a measure of pomegranate juice authenticity and was detected in the dataset. Additional analysis using ion mobility enabled QTof was performed and shown to be capable of separating this isomeric pair (data not shown).
Here we have shown benefits of SONAR for the application of fruit juice profiling for QC and authenticity purposes including:
Ion mobility enabled QTof-MS analysis was also employed to facilitate isobaric marker identification and ratios in complex juice samples.
720006468, February 2019