
In this application note, we examine how mass detection with the ACQUITY QDa can be used to more reliably detect parthenolide in feverfew ingredients and finished products.
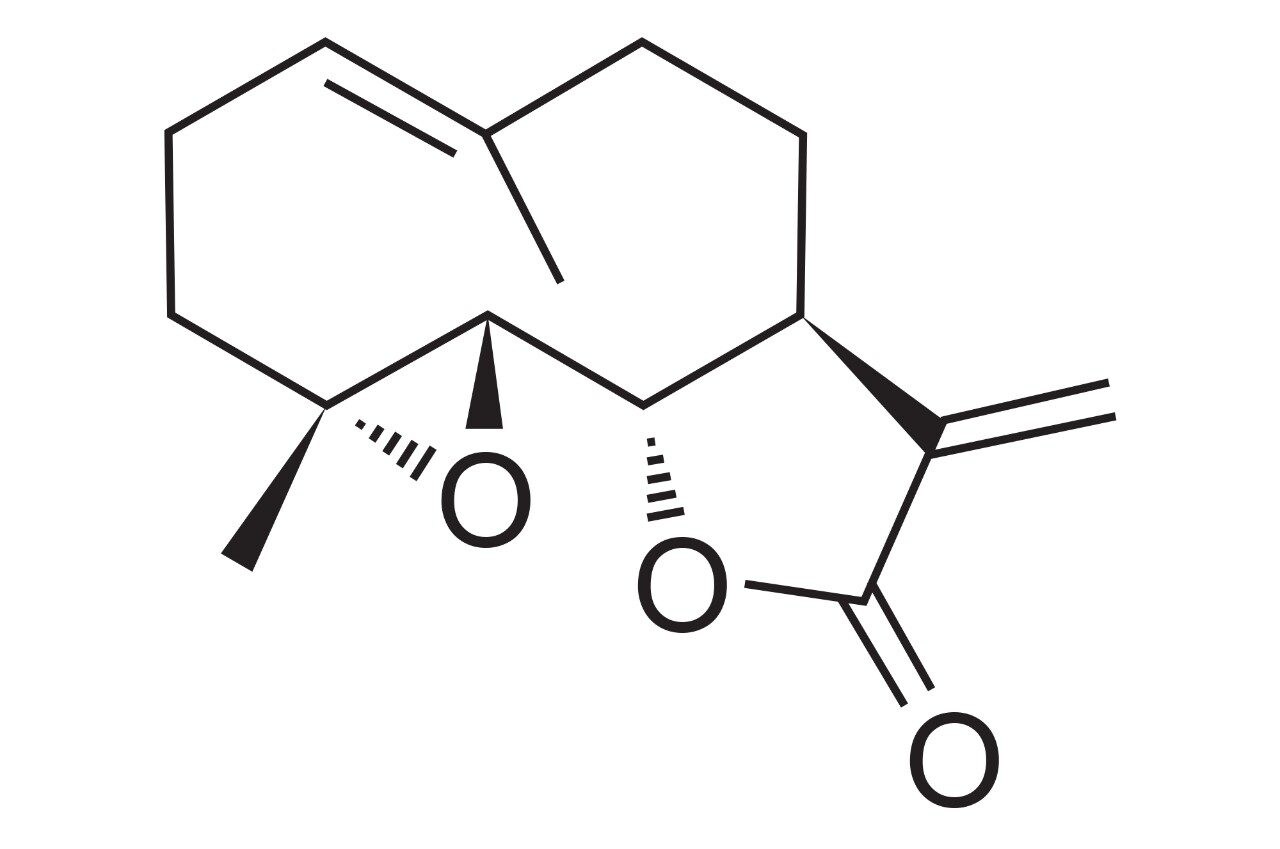
Feverfew (Tanacetum parthenium) (Figure 1) is a medicinal herb that has long enjoyed traditional use as a remedy for migraine headache pain. The above ground or aerial parts of feverfew contain the sesquiterpene lactone parthenolide (Figure 2), which is widely believed to be responsible for the plant's biological activity.1 Dried feverfew aerial parts should contain approximately 0.2–0.5% of parthenolide by weight and adults are recommended a dose of 0.2–0.6 mg parthenolide daily for migraine prophylaxis.2,3 As a result, manufacturers of feverfew herbal supplements require robust and accurate methods capable of detecting and quantifying low levels of parthenolide in feverfew ingredients and finished products.
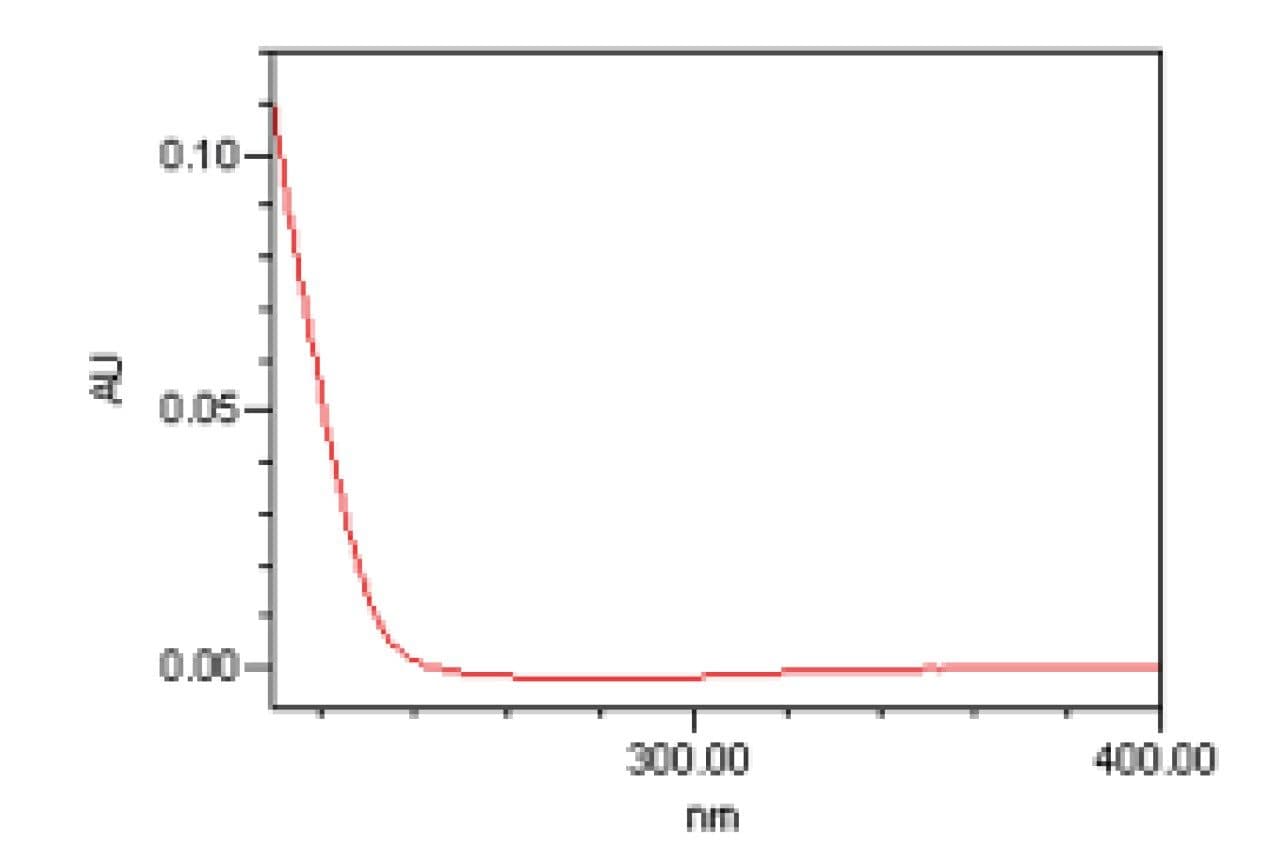
Liquid chromatography with UV detection is widely used in the supplement industry for the analysis of plant materials and herbal supplement products. While many naturally occurring compounds respond well to UV detection, there are classes of compounds (such as the sesquiterpene lactones including parthenolide (Figure 3)) which demonstrate poor UV absorbance. In analytical methods, these compounds are often detected using low wavelengths (200–220 nm). When low wavelengths are used for measurement, the UV detector responds to nearly all compounds present in a sample and lacks specificity for any single compound. While such methods may be fit for purpose for testing relatively simple or highly purified samples with low or minimal matrix, they are often unsuitable for the analysis of complex samples, such as herbal ingredients and herbal supplement products, due to interferences from other components present in the sample.

Sample cleanup or enrichment (such as solid-phase extraction) and rigorous method development could both be employed to minimize the effect of interferences and ensure accurate identification and quantification, however these approaches require additional time and cost. Additionally, the chemical composition of plants is well-known to change over time (due to factors such as growing conditions, geographic location, plant maturity, post-harvest processing, etc.) and methods used to address interferences today may not work equally well for tomorrow’s samples. Instead, an analytical method which is more specific to the analyte of interest is the best solution for ensuring long-term method performance for a wide variety of materials.
In this application note, we examine how mass detection with the ACQUITY QDa can be used to more reliably detect parthenolide in feverfew ingredients and finished products.
The method was adapted from Avula et. al4 with some modifications. Samples of authenticated dry feverfew aerial parts were provided by the University of Mississippi National Center for Natural Products Research. Two herbal dietary supplements containing feverfew herb were purchased at a local store. Samples were extracted by adding 10 mL of acetonitrile to 500 mg of each sample followed by sonication for 30 minutes. Sample solutions were allowed to settle and then filtered through a 0.45 μm PTFE syringe filter. Each solution was then diluted 1:100 or 1:1000 with acetonitrile prior to analysis. Standard solutions of parthenolide (Sigma-Aldrich # P0667) were prepared in acetonitrile.
|
LC system: |
ACQUITY UPLC H-Class (operated as an HPLC) |
|
Detection: |
ACQUITY PDA eλ and ACQUITY QDa (Performance) |
|
Column: |
Waters XBridge BEH C18 150 x 4.6 mm, 5 μm (P/N 186003116) |
|
Column temp.: |
50 °C |
|
Sample temp.: |
25 °C |
|
Injection volume: |
10 μL |
|
Wavelength: |
217 nm |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Methanol |
|
Time |
Flow Rate (mL/min) |
%A |
%B |
|---|---|---|---|
|
0.0 |
1.0 |
80 |
20 |
|
30.0 |
1.0 |
10 |
90 |
|
35.0 |
1.0 |
80 |
20 |
|
40.0 |
1.0 |
80 |
20 |
|
MS system: |
ACQUITY QDa (Performance) |
|
Ionization mode: |
ESI+ |
|
Cone voltage: |
10 V |
|
Sampling rate: |
1 points/sec |
|
Mass range: |
100–600 Da |
|
MS experiments: |
SIR (249.1 Da) |
|
Smoothing: |
5 point mean |
|
Data management: |
Empower 3 FR 2 CDS |
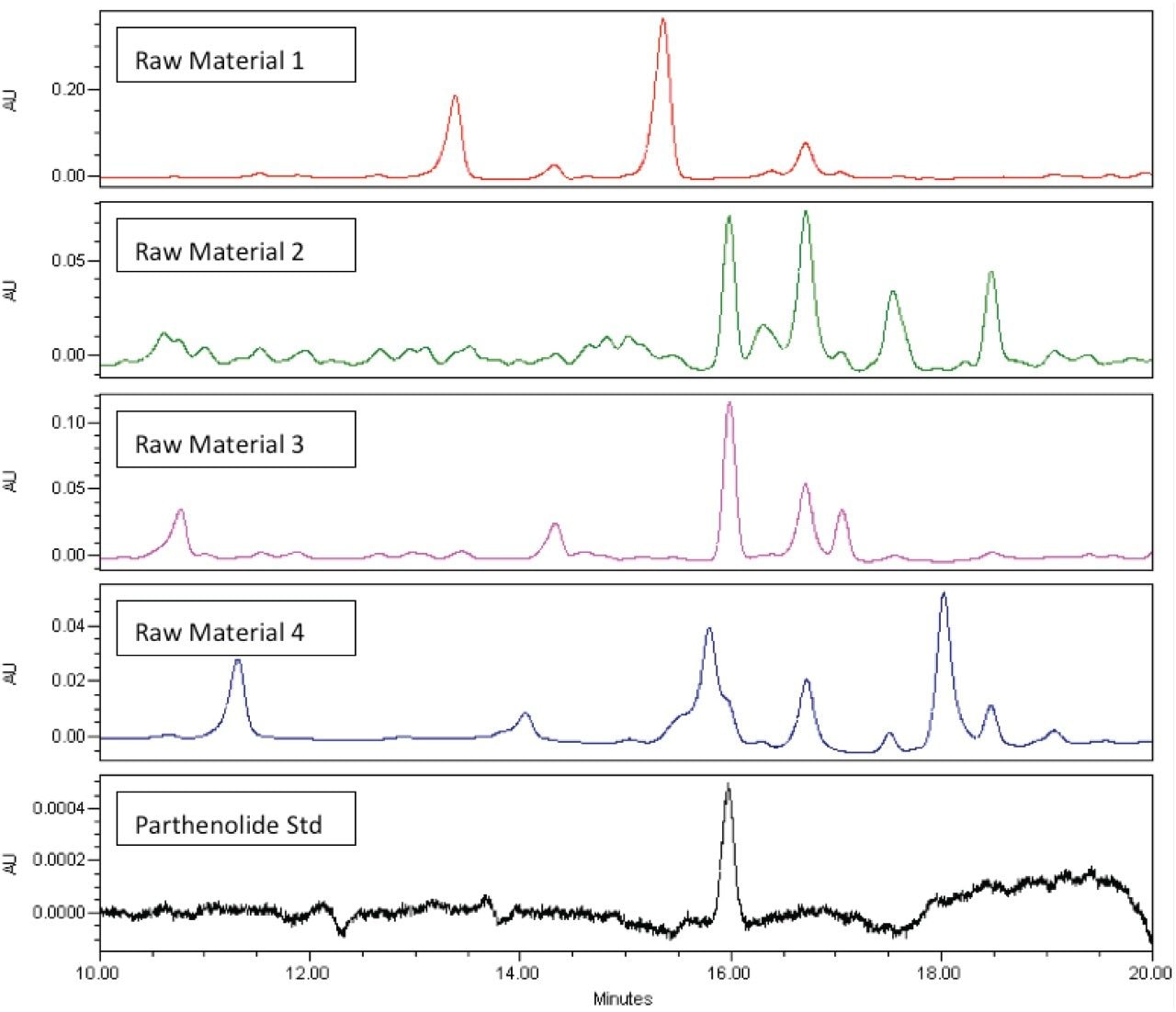
Four different lots of feverfew aerial parts were analyzed by HPLC/UV (at 217 nm) and the resulting UV chromatograms are shown in Figure 4. While it is clear that Raw Material 2, 3, and 4 do contain parthenolide, there are a variety of other components in each chromatogram that appear unique to that sample. Although parthenolide is well-resolved from neighboring peaks in Raw Material 2 and 3, we find that a naturally-occurring compound in Raw Material 4 nearly co-elutes with parthenolide and prevents accurate detection and quantification of the analyte of interest.
As mentioned previously, use of low UV wavelength detection (200–220 nm) results in a non-specific detector response and increases the likelihood that other components in the sample will interfere with detecting the analyte of interest. This can be particularly problematic when such methods are applied to complex samples or multi-ingredient herbal supplements. Methods (such as this one) which work well for only some samples and materials are inappropriate for routine use in the QC environment.
One popular alternative for detecting compounds with poor UV absorbance is mass detection, however the size, cost, and complexity of traditional mass spectrometers has limited their use in the QC environment. The ACQUITY QDa was designed to integrate seamlessly into the chromatographic workflow and can be added easily to an existing HPLC or UPLC instrument, providing mass data in addition to UV data.
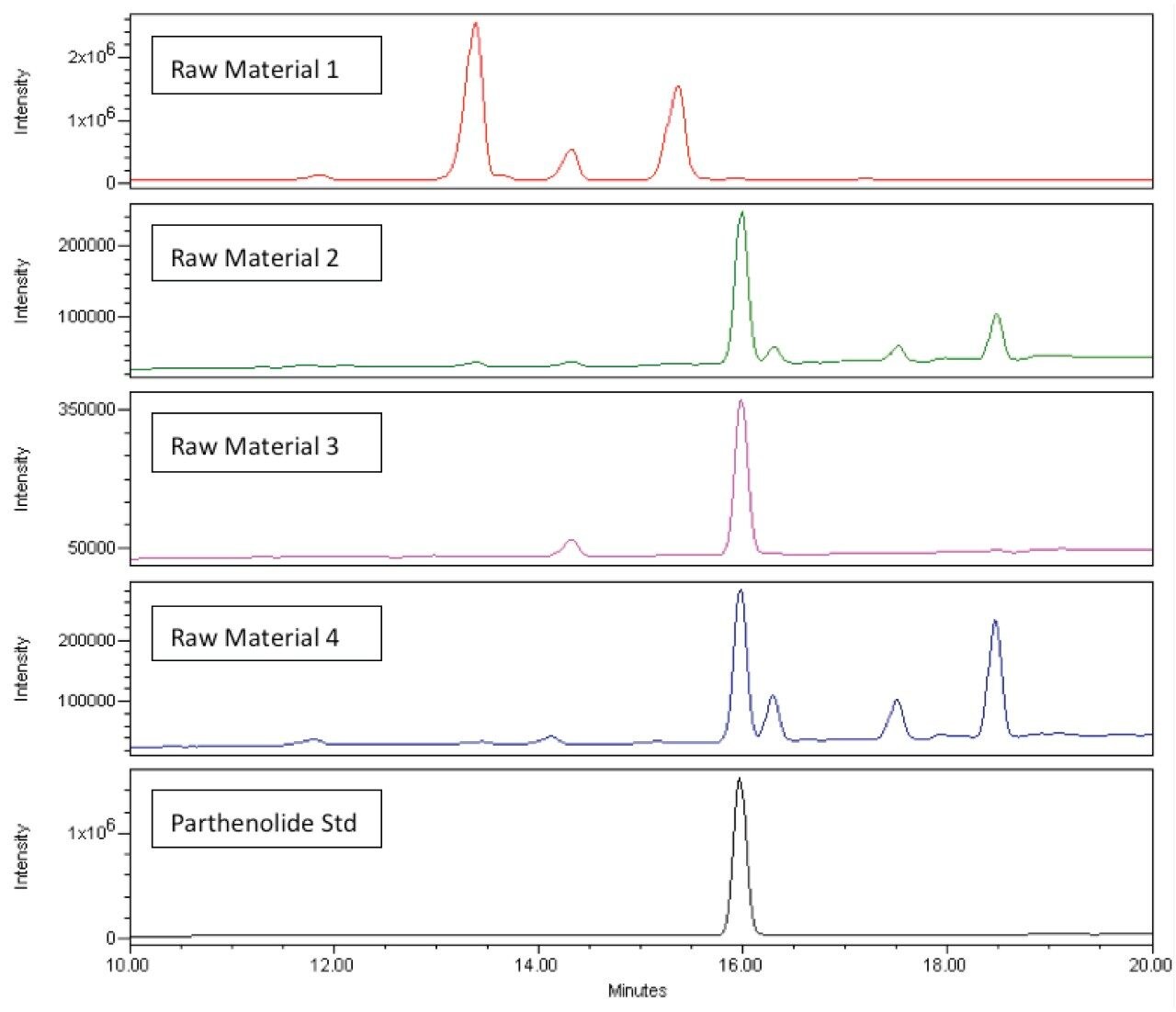
The separation and detection of the same four lots of feverfew raw material is shown in Figure 5. With the addition of mass detection, we have baseline resolution of close-eluting compounds to parthenolide and the interference present in Raw Material 4 has been eliminated.
Methods used for QC testing must work not only for individual raw materials but must also be capable of detecting and measuring the analyte of interest in finished supplement products. Two multi-ingredient herbal supplements for migraine relief were analyzed using traditional UV detection and the ACQUITY QDa.
As shown in Figure 6 (below) parthenolide is easily detected in Product A, however it appears that an interference present in Product B co-elutes with parthenolide. Review of the UV spectrum for the peak at ~16.0 minutes confirms that the UV spectrum of the peak does not match the reference spectrum for parthenolide.
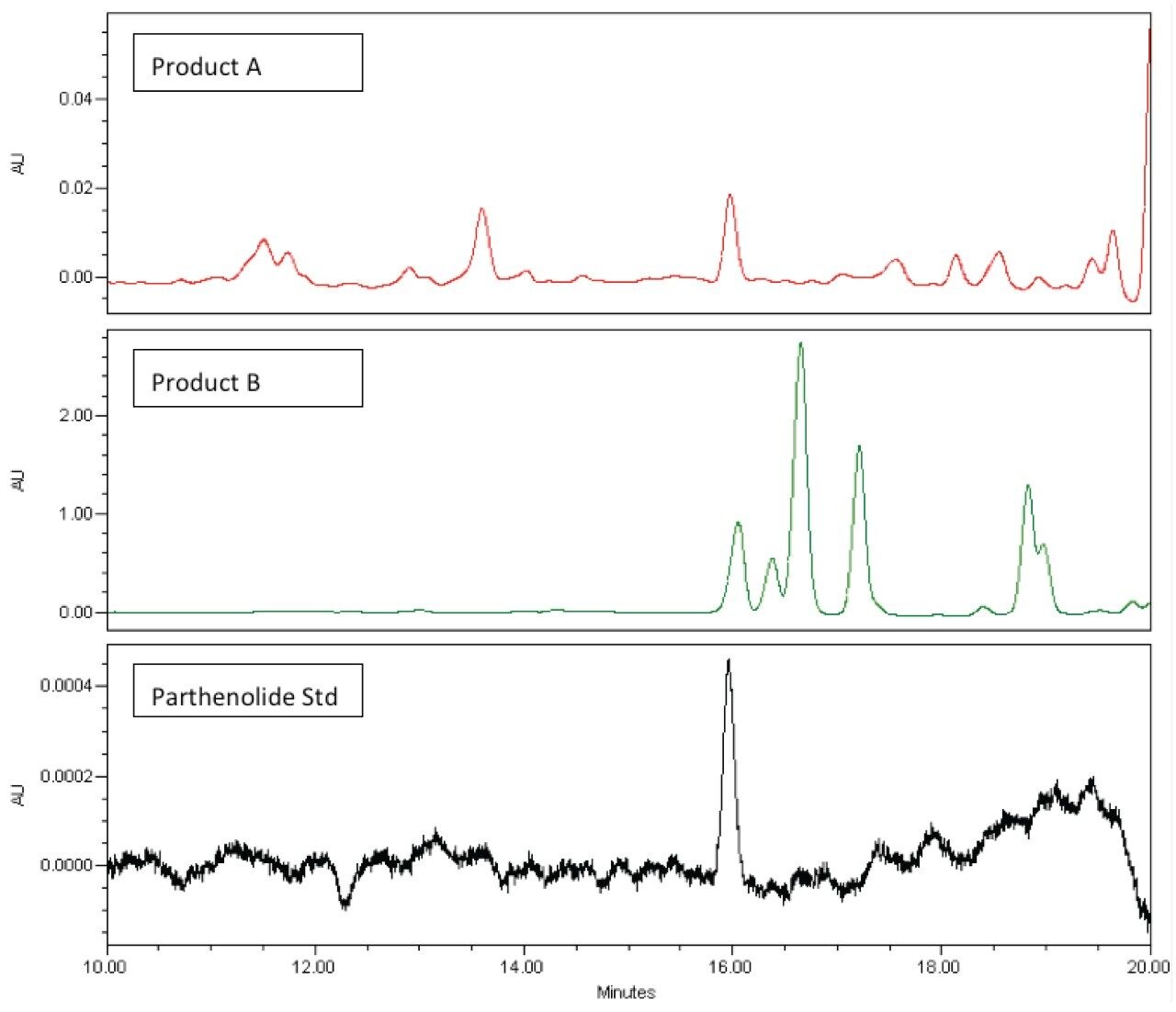
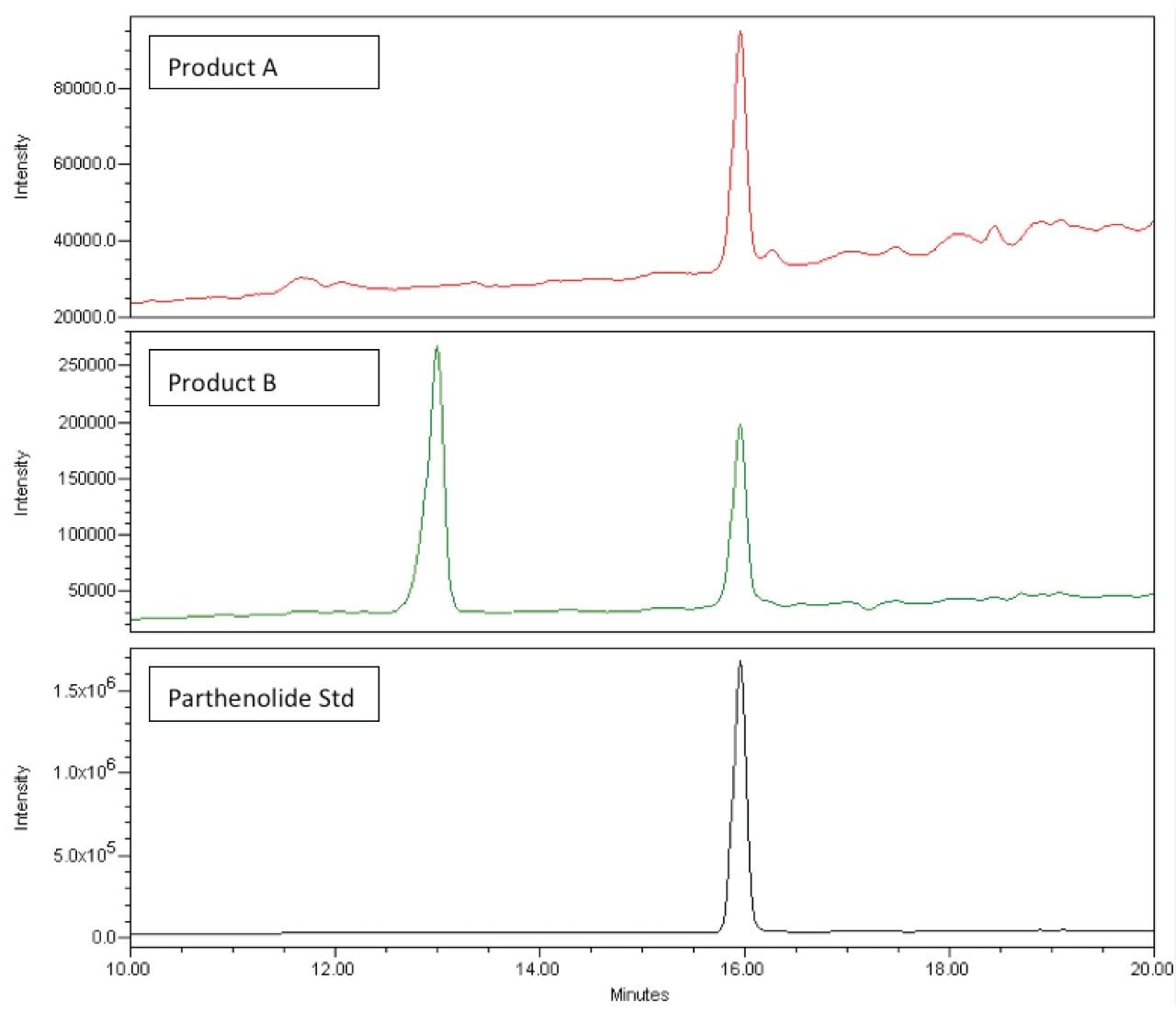
Using the mass detection capabilities of the ACQUITY QDa, we again see that the interfering peak is eliminated and parthenolide can be easily detected in both Product A and Product B (Figure 7).
Further inspection of the peak at 16.0 minutes indicates that the interfering peak has a mass of 275 Da. Eventually this peak was identified as methysticin (a kavalactone found in Kava Kava root which is one of the main ingredients in this product).
Separately plotting the mass response for 249.1 Da (partheonlide) and 275.1 Da (methysticin), we see that these compounds are slightly resolved by retention time (Figure 8).

Methods used in the QC laboratory should be capable not only of qualitatively detecting the presence of target compounds, but should also be useful quantitatively for determining the amount of target analyte in both raw materials and finished products.
Prior application notes5-7 demonstrate that the ACQUITY QDa mass detector can indeed be used for quantitative applications. In this study, a calibration was also performed and all raw materials and finished products assayed for parthenolide content.
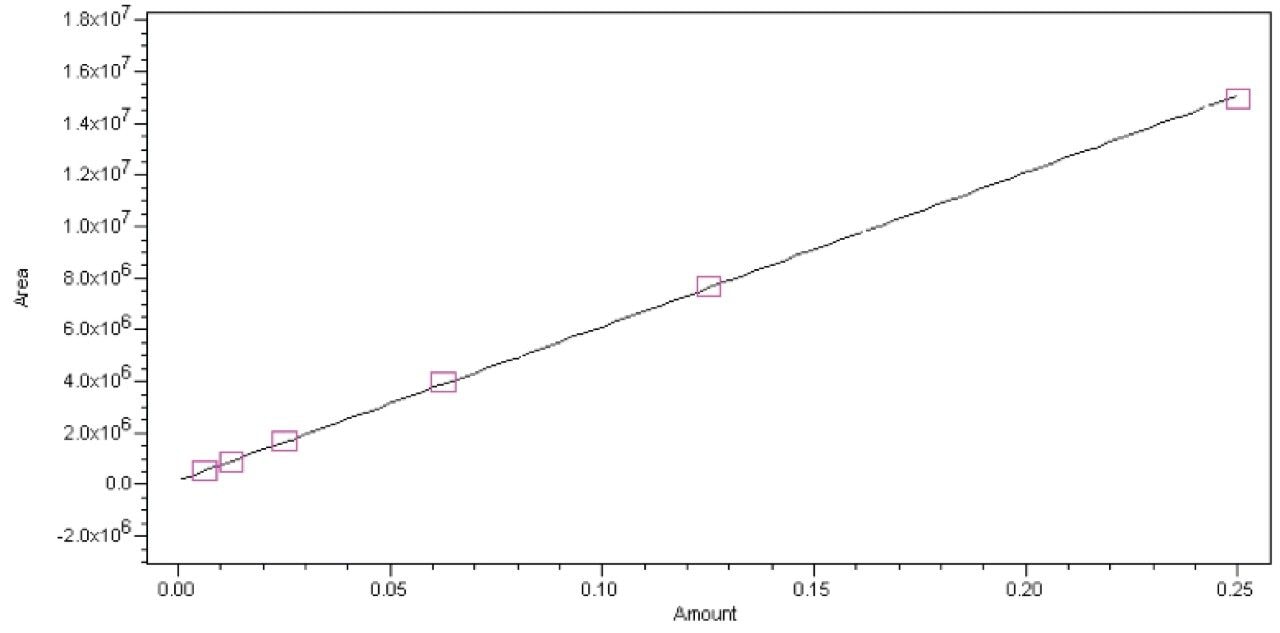
A dilution study was first performed to identify the linear operating range of the ACQUITY QDa mass detector and then six non-zero calibration standards over the range of 6.25–250 ng/mL were prepared and analyzed. The curve demonstrated excellent linearity (0.9999) and is shown in Figure 9.
Samples were assayed using external calibration and a weighting of 1/X. Results are shown in table 1.
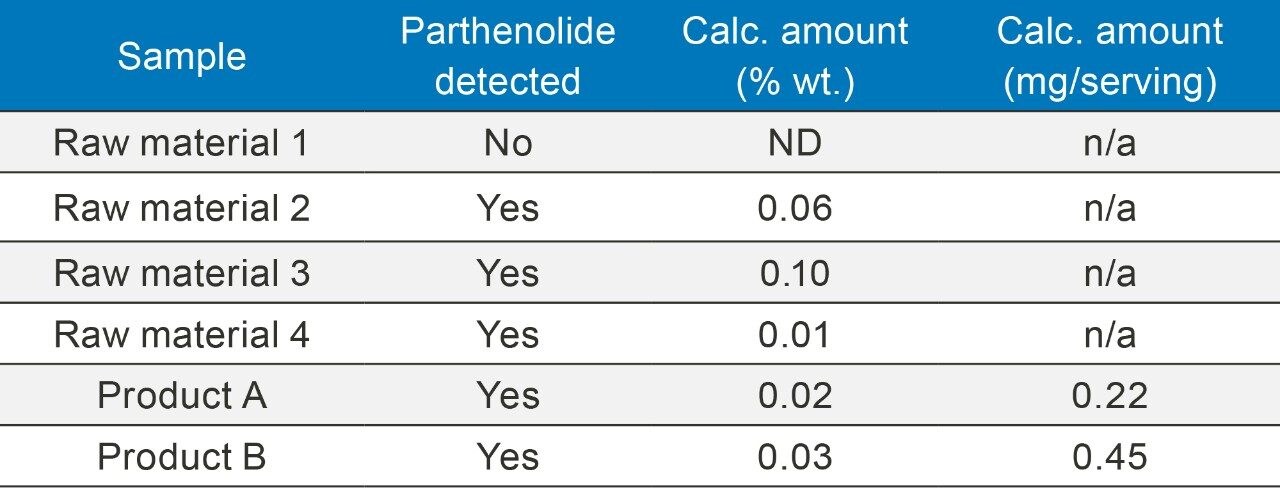
HPLC/UV detection is widely used for detecting and quantifying compounds in plants and herbal supplements. While this technique works well for compounds with strong UV absorbance, using low-wavelength detection for poor UV absorbers can be problematic. Interferences inherent to the sample or introduced by other ingredients can prevent accurate detection and quantification of target analytes and such assays cannot be used reliably to satisfy regulatory requirements for QC testing. The ACQUITY QDa Mass Detector provides an alternative approach to detecting and quantifying compounds with poor UV absorbance and results in methods with improved sensitivity and better specificity.
*This work was completed while James Traub held a position at Waters Corporation.
720006206, February 2018